Scientists design a star-shaped dianion only to discover it had probably been synthesised nearly two decades ago
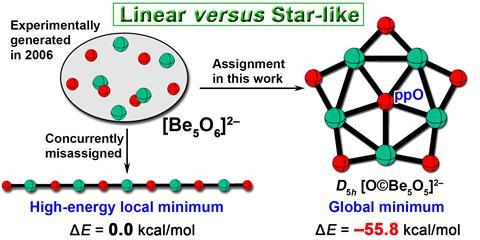
Scientists in China have theorised a star-shaped beryllium-based dianion, [O©Be5O5]2−, which holds the first example of a stable planar pentacoordinate oxygen within a small multiply charged anion, meaning the central oxygen is bonded to five beryllium atoms in a flat plane.1 Computational analysis indicates that the unique structure is stable as the central oxygen satisfies the octet rule and oxygen to beryllium π-backbonding stabilises the electron-deficient beryllium atoms.
Small multiply charged anions are notoriously difficult to generate in the gas phase due to their tendency to lose electrons. Over the years, computational studies have predicted structures with planar pentacoordinate configurations, but no experimental reports of planar pentacoordinate clusters exist and oxygen-centred planar pentacoordinate clusters were completely unexplored. This gap in the literature prompted Yan-Bo Wu and Caixia Yuan from Shanxi University and colleagues to wonder if they could design a stable [Be5O6]2− cluster.
A few years ago, the team developed an electron compensation strategy to design hypercoordinate motifs thought too reactive to isolate.2 The strategy involved introducing electron-rich non-metals into motifs containing electron-deficient atoms to stabilise the structure and protect the cluster core. Using this electron-compensation strategy as a springboard, the team proposed the star-shaped [O©Be5O5]2− cluster and used computational methods to establish its stability.
While developing the structure, they stumbled across a 2006 experimental study of a small multiply charged anion with the same molecular formula, [Be5O6]2−.3 The same year, a corresponding computational study by Andreas Dreuw, when he was at Goethe University Frankfurt in Germany, predicted [Be5O6]2− would have a linear structure with alternating oxygen and beryllium atoms.4
The Shanxi team’s analysis included exploring the potential energy surface of possible molecular configurations using a stochastic search algorithm. Calculations showed that their planar pentacoordinate oxygen star-shaped structure is the global energy minimum, 55.8kcalmol -1 more stable than the linear isomer, disproving Dreuw’s computational prediction.
Dreuw, who’s now at Heidelberg University in Germany, says he was surprised by the peculiarity of the Shanxi team’s structure: ‘But when you think about it, then it’s immediately clear that this should be stable.’ Advances in computational tools mean scientists can now efficiently search for minima across the full potential energy surface, something that was unrealistic back in 2006. ‘Now you have programs where you can sample whole ensembles … and find structures that you had not expected, such as this one,’ Dreuw adds.
What’s more, it means that this apparently unprecedented planar pentacoordinate oxygen in a small, multiply charged anion might actually have been made experimentally nearly 20 years ago. ‘It created that rare eureka moment … [and] demonstrated how fundamental chemical principles can remain hidden in plain sight within existing data,’ says Wu.
The Shanxi team also investigated the electronic structure of the dianion. Analysis confirmed that the central oxygen is a dianion and that the cluster contains five beryllium–oxygen–beryllium π bonds. These bonds form the outline of the star shape and add stability to the Be5O5 framework as oxygen to beryllium π-backbonding compensates for the electron deficiency of the beryllium atoms. The star-shaped Be5O5 skeleton perfectly accommodates the central oxygen dianion, creating an electrostatic sandwich with a negatively charged central oxygen, a positively charged inner ring and a negatively charged outer ring. Analysis into the interactions between the central oxygen and the beryllium skeleton showed that electrostatic interactions dominate, accounting for 71.9% of the attractive energy. Wu says was an additional surprise for the team: ‘We’ve always thought covalent bonds are the main stabilising factors in planar hypercoordinate carbon clusters – this is almost textbook… [so] this discovery really turned our initial assumptions on their head.’
References
1 R Sun et al, Chem. Sci., 2025, DOI: 10.1039/d5sc02361k
2 B Jin et al, Chem. Commun., 2022, 58, 13095 (DOI: 10.1039/d2cc05654b)
3 K Franzreb and P Williams, Chem. Phys. Lett., 2006, 419, 379 (DOI: 10.1016/j.cplett.2005.11.106)
4 A Dreuw, Chem. Phys. Lett., 2006, 419, 385 (DOI: 10.1016/j.cplett.2005.11.107)











No comments yet