Computational research has unpicked the stability of a trivalent oxygen species that forms in oxygen-doped graphene. This fresh understanding could guide scientists designing carbon-based nanomaterials.1
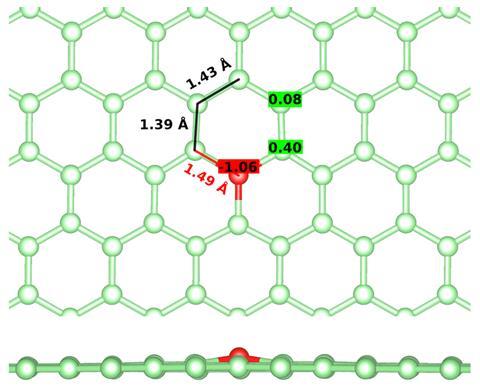
In 2019, researchers in Germany and Austria analysing oxygen-doped graphene with aberration-corrected scanning transmission electron microscopy and single-atom electron energy loss spectroscopy observed oxygen atoms connected to three carbon atoms.2 This new and unexpected bonding configuration contradicted the textbook notions of oxygen forming one double or two single bonds.
Other exceptions are known. A trivalent form of oxygen called oxonium, for example, features in several molecules forming positively charged states. Oxonium ions are highly reactive. The trivalent oxygen in oxygen-doped graphene, however, was surprisingly stable and stood up to the invasive imaging methods. And while they could tell it was planar, or quasi-planar, and not positively charged (unlike oxonium ions), a consistent theoretical explanation of its bonding mechanisms was lacking.
Now, a different team of researchers has modelled the interactions surrounding oxygen-doped graphene’s oxygen species to rationalise its stability. Their calculations mixed periodic density functional theory with the adaptive natural density partitioning method.
The calculations revealed the oxygen is bonded to its three nearest carbon atoms through C–O σ-bonds, and π-bonds with both bonding and antibonding character. They identified local π-aromaticity, with a delocalised 4c–2e bond, as the primary source of stability for the graphitic oxygen species. Aromaticity also explains the extended planar structure of oxygen binding to three carbon neighbours.
By optimising the structure of the trivalent oxygen species, the team then proposed four stable planar molecules: [OC36H15]13+, [OC72H21]19+, [OC120H27]25+ and [OC180H33]31+. These theoretical molecules demonstrate how unveiling the factors driving the stabilisation of oxygen in this bonding configuration can unlock the design and properties of new families of molecules.
‘The contribution is important to deepen our understanding of the impact of oxygen in carbon-based nanomaterials, oxidised carbon nanotubes or graphene. This is especially relevant considering that oxygen is usually present in the fabrication of these materials. Moreover, it can be key in the study of organic molecules, which are two-dimensional and π-conjugated,’ reflects Elisa Jimenez-Izal from the University of the Basque Country in Spain, who co-led the study.
‘The next steps will be to determine the applications that this exotic species could enable. We would like to explore the potential catalytic properties of this system,’ says Jimenez-Izal. ‘With respect to the optical properties, the horizon is also full of possibilities. These impurities might lead to excitons, in the same way as nitrogen-doping can behave in graphene.’
Theoretical chemist Ramon Quintana Miranda, from the University of Florida in the US, comments that the work ‘opens the door to tuning the properties of graphene-like materials in new and exciting ways. The authors provide a comprehensive analysis of the factors dictating the stability of these compounds, which can in turn be used by the experimentalists as design principles to explore new conformations in these materials.’
References
1 A Ugartemendia et al, Chem. Sci., 2024, 15, 6151 (DOI: 10.1039/d4sc00142g)
2 C Hofer et al, Nat. Commun., 2019, 10, 4570 (DOI: 10.1038/s41467-019-12537-3)








No comments yet