Colloids are everywhere that we look, so why is it that most people know so little about them, asks Mike Garvey
Colloids are everywhere that we look, so why is it that most people know so little about them, asks Mike Garvey
I was fortunate to be introduced to colloid science as an undergraduate in 1968 by two enthusiastic lecturers, Duncan Shaw and Alec Smith. Duncan Shaw had recently written the first undergraduate level introduction to the subject which, now in its fourth edition, remains a valuable stepping-stone for those entering the field. Although Michael Faraday, one of the founders of colloid science, had beaten us to it by more than a century, as students we saw for ourselves the fascination of gold sols, nanometre-sized particles of gold dispersed in water as a stable colloid with a red coloration which changes to blue on adding salt. Gold sols have currently gained renewed interest as a building block for nanotechnology.
After graduating, I began working for ICI, initially on chlorochemicals for printing inks, PVC and weedkillers, before transferring to agrochemicals, investigating the formulation of pesticides as solid-in-liquid dispersions or emulsions for crop spraying. After 10 years with ICI, I joined Unilever Research where I remained for 25 years, applying colloid science to such diverse subjects as effluent treatment, fabric washing tablets, pregnancy test kits, paper making and lubricants for coal mining.
Throughout my career what has been striking is the impact of colloid science in so many areas of industrial chemistry, evidenced by the strong colloid science base of companies such as BP, Shell, Unilever, ICI, Procter & Gamble, Kodak and many others. Yet despite its importance, it still surprises me that colloid science remains a subject that is glossed over in most chemistry degrees.
Defining ideas
Colloid means glue-like, originating from the Greek,
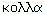
.
It describes materials that are predominantly liquid but which have other properties: either optical, giving rise to turbidity such as milk, or viscous, with characteristics of mucus, gelatin or wet clay. These effects arise from the presence of macromolecules dissolved in liquid and/or by mixing two or more solid, liquid or gas phases.
Colloid science can therefore be described on the one hand as the study of solutions of macromolecules, for example proteins in water or solutions of synthetic polymers, such as the clear glues for model construction kits. On the other hand it is the study of dispersions of one phase in another, for example emulsions (oil in water or water in oil), solid in liquid, foams and the complex lyotropic liquid crystal dispersions of soap or synthetic detergents. Some readers will remember the old problem of mushy soap bars when left in contact with water, which arises from the ingress of water expanding the once hard compacted soap.
Alternatively, colloid science is the study of systems in which one or more dimensions is in the range of approximately 1nm to 10?m. For example, synthetic polymers with a molecular weight of 100,000 adopt a random coil dimension of ~40nm in solution, emulsion droplets are typically 1 to 10?m in diameter, and foam films are typically 10 to 100nm thick.
Colloidal dispersions
Colloidal dispersions are two-phase systems comprising the dispersed phase and the continuous or dispersion medium. The mixture is homogeneous over an appreciable time period. This period will depend on the application; precipitated metal hydroxides for potable water treatment are purposely short-lived in stability in order to flocculate and remove the natural organic matter. By contrast, domestic dispersions such as paint and abrasive cleaners will have a shelf-life of several months or years. The properties of the dispersion are determined to a large extent by the nature of the dispersed phase-dispersion medium interface. Colloid science and surface science are therefore closely linked.
The selected examples shown in Table 1 illustrate the scope and importance of colloidal dispersions.
Table 1. Some colloidal dispersions
| Dispersed phase | Medium | Type | Examples |
| Liquid | Gas | Aerosol | Fog, sprays |
| Liquid | Liquid | Emulsion | Salad dressing |
| Liquid | Solid | Solid emulsion | Pearl, opal |
| Solid | Solid | Solid suspension | Pigmented plastics |
| Solid | Liquid | Sol or paste | Ink, toothpaste |
| Solid | Gas | Aerosol | Inhalers, smoke |
| Gas | Liquid | Foam | Fire extinguisher, detergent foam |
| Gas | Solid | Solid foam | Pumice stone, expanded polystyrene |
In general, emulsions and sols are termed lyophobic colloidal dispersions. Lyophobic (liquid hating) dispersions cannot be prepared by dissolving the dispersed phase in the dispersion medium. They are made by one of two methods.
(1) Condensation methods, in which molecules combine in solution to form a precipitate of colloidal dimensions. Examples include the precipitation of silver halides from soluble salts, the technology that underpins the photographic industry. Indeed, much of the fundamental understanding of colloid stability stems from the detailed investigation of silver halide sols, particularly by the Dutch colloid schools. Another example is the reduction of chlorauric acid to form gold sols.
(2) Dispersion methods, where one phase is dispersed in the other by mechanical means. Examples include comminution, by which large particles in a liquid medium are broken down by mechanical force, using a colloid mill with an abrasive rotor and stator, a ball mill using the cascading action of hard ceramic balls, or a bead mill in which the beads and particles are sheared followed by separation of the dispersion. Emulsification is generally achieved using a homogeniser in which oil and water are forced, under pressure, through an adjustable orifice.
Lyophilic (liquid loving) colloids describe solutions of macromolecules. Unlike lyophobic colloids, which are two-phase systems, lyophilic colloids are true solutions. Both lyophobic and lyophilic colloids confer similar properties to the liquid such as gel formation, shear thinning and shear thickening (changes in viscosity on shaking and stirring). To understand colloidal dispersions requires a sound appreciation of both systems since many applications use combinations of lyophobic and lyophilic colloids. For example, polyelectrolytes aid the flocculation of metal hydroxides for water treatment and polymeric stabilisers prevent salt-induced aggregation of gold sols.
Colloid stability
A colloidal dispersion of sub-?m particles may be stable or unstable to aggregation. Brownian motion ensures that the particles are in continual motion, giving rise to collisions at a rate determined by diffusion theory. Owing to the high interfacial free energy, lyophobic colloids are thermodynamically unstable and tend to aggregate. This is generally undesirable and colloid scientists aim to prevent it from occurring.
In a stable dispersion the particle collisions do not lead to aggregation because inter-particle repulsion forces dominate. It will remain dispersed indefinitely, although particles bigger than about 0.1?m will sediment depending on their density. In an unstable dispersion, the collisions lead to aggregate formation; larger aggregates either sediment or cream depending on their relative density.
The repulsive forces in a stable dispersion were long ago identified as being electrical in origin. A surface potential exists at the interface between the solid particle and the surrounding liquid due to the presence of a surface charge. To maintain electrical neutrality, ions of opposite charge present in the medium are attracted closer to the particle surface, resulting in a diffuse layer of highly concentrated counterions. The concentration of counterions in this layer decays exponentially from the surface over a distance of tens of nanometres. The resulting ionic cloud is called the diffuse region of the electric double layer. On particle-particle collision, overlap of the ionic clouds gives rise to an osmotic repulsion that pushes the particles apart.
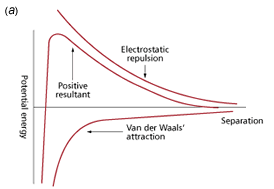
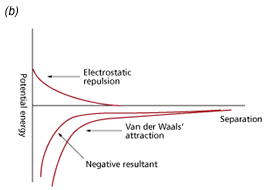
The DLVO theory of colloid stability, developed by Derjaguin and Landau, and Verwey and Overbeek during the 1940s proposes a balance of the repulsive electric double layer forces (positive by convention) and the attractive van der Waals’ forces (negative by convention) that exist between all matter. These two forces were found to be of similar range and magnitude. The electrical forces increase exponentially as particles approach one another and the attractive forces increase as an inverse power of separation. As a consequence, these additive forces may be expressed as a potential energy versus separation curve. A positive resultant (Fig 1a) corresponds to an energy barrier and repulsion, while a negative resultant (Fig 1b) corresponds to attraction and hence aggregation. It is generally considered that the basic theory and its subsequent modifications provide a sound basis for understanding colloid stability.
Fig 1. Potential energy curves for stable and unstable dispersions
The adsorption of lyophilic colloids - macromolecules - by the surface of lyophobic colloids gives rise to an additional repulsive force. Macromolecules attach to the surface to form a loop-like configuration of trains of segments attached to the surface, and loops and tails of segments extending out into the liquid phase. Research, mainly during the 1960s and 1970s, identified the nature of the repulsive forces arising from such adsorption. These are a combination of entropic repulsion, arising from the restricted configurational freedom of the adsorbed molecules when two particles collide; and osmotic repulsion, arising from the increased concentration of segments in the overlap region of the adsorbed layers on particle-particle contact.
Except under special conditions, the presence of a saturated adsorbed layer always leads to a total stabilisation of the dispersion to coagulation. Earlier publications referred to this effect as colloid protection but it is now termed steric stabilisation.
Common stabilisers are the polymeric dispersants used in formulating printing inks to ensure that the dispersed phase remains as discrete units. Whole industries are built around the design and selection of dispersants to optimise product performance. Invariably, together with other macromolecules or liquid crystalline surface-active agents, these agents modify the flow characteristics of the product. The formulator’s skill lies in achieving the necessary product characteristics such as mouth-feel (organoleptic properties) with synthetic foodstuffs, non-drip properties of paints and prevention of fine mist with liquid aerosols. The list of uses is endless; Table 2 lists just a few of the industrial applications.
Table 2. Some industrial applications of colloids
| Effluent treatment | Precipitation and/or flocculation for clarification |
| Paint industry | Achieve homogeneous films, toughness and ’hiding’ power |
| Food industry | Stable creams and gels |
| Cosmetics and toiletries | Emulsions, toothpaste |
| Detergent industry | Stabilisation of suspended soil, liquid abrasives |
| Pharmaceutical industry | Stable dispersions to ensure uniform dose of active drug |
| Agricultural industry | Pesticides formulated as dispersions |
This brings me back to the title of this article: The impact of colloid science. In recognition of the importance of colloids to so much of the UK’s industrial base, Impact, (Innovative Materials Development and Product Formulation by the Application of Colloid Technology) is the new Faraday Partnership that is funded by the Department of Trade and Industry and the Engineering and Physical Sciences Research Council.
Impact is helping to coordinate colloid research between industry and the academic base and, in recognition of the ongoing need for training in colloids, the partnership is currently developing a distance-learning course for the internet. This will help future generations of scientists not only to recognise the importance of colloid science, but also to promote its successful application.
Source: Chemistry in Britain
Acknowledgements
Mike Garvey is a technology translator for the Impact Faraday Partnership and a senior research fellow in University of Liverpool.
Further Reading
- D. F. Evans and H. Wennerstrom, The colloidal domain, 2nd edn. Weinheim: VCH, 1999.
- D. J. Shaw, Introduction to colloid and surface chemistry, 4th edn. London: Butterworth-Heinemann, 1992.
A taste of food colloids
It is highly likely that you regularly consume some sort of colloidal food. Colloids are ubiquitous in Western diets - from the milk that you pour on your breakfast cereal and the margarine that you spread on your toast, to the dollop of mayonnaise that enriches your sandwich and the dressing that you drizzle over your salad.
Most food colloids are emulsions: milk, cream and mayonnaise are oil-in-water emulsions while margarine and low-fat spreads are water-in-oil. Without emulsifiers (surface-active agents) to protect droplets and stop them from merging together, emulsions would soon separate into their constituent phases. Two broad classes of emulsifiers are added to foods: low-molecular weight emulsifiers (eg monoglycerides, lecithin and polysorbates) and macromolecular emulsifiers (usually egg or milk proteins). Proteins are particularly good emulsifiers, imparting stability through both electrostatic and steric mechanisms.
Even with a good emulsifier, an emulsion is potentially unstable because droplets tend to settle under gravity, causing sedimentation (if the droplets fall) or creaming (if the droplets rise). In general, oil-in-water emulsions cream, as commonly seen in milk, whereas water-in-oil emulsions sediment. Creaming and flocculation will almost always occur, to some extent, in food colloid systems, but they are generally tolerated because they are reversible and a gentle stir will solve the problem. Adding stabilisers such as high molecular weight polysaccharides (eg carrageenan or xanthan) extends the shelf life of colloidal foods. These increase the viscosity of emulsions, reducing droplet movement and slowing down the process of creaming or sedimentation.
Another way to increase stability is to reduce the size of emulsion droplets. For example, manufacturers of cream liqueurs need to ensure that less than 3 per cent of the emulsion droplets have droplet diameters exceeding 0.8?m. The inevitable small amount of creaming that takes place over prolonged storage periods is hidden behind coloured glass and strategically placed labels on the bottle necks.
Foams also require surface-active agents for stability. For example, the head on a pint of beer is primarily stabilised by adsorbed proteins and polypeptides derived from proteins in the malt. The ethanol present in beer helps the foam to form because it sharply lowers surface tension, producing smaller gas bubbles. As anyone who bakes cakes is aware, egg white is a very effective foaming agent. Its success can primarily be attributed to proteins and glycoproteins in chicken egg albumen, which stabilise the air bubbles that are whisked in.
Classifying food colloids is not always easy because most are complex multiple systems. For example, ice cream is an emulsion, a foam and a dispersion, comprising fat droplets, air cells and ice crystals dispersed in an aqueous continuous phase and aggregated into a semi-solid frozen aerated matrix (see Chem. Br., July 2001, p62). Mmm...sounds delicious.
Emma Davies
Colloid stability
Shown below are electron micrographs of the dried sediment from a latex of spherical polystyrene particles ~300nm diameter. The sediment on the left has been formed from a colloidal dispersion in which the particles have been modified to give a strong inter-particle repulsion. The particles glide around each other after sedimentation, rather like lubricated ball bearings in a bucket. The result is a close-packed sediment. By way of the inter-particle spacing, ~300nm, the sediment displays iridescent colours akin to that of opal.
The sediment on the right has been formed from a colloidal dispersion in which the particles have been modified to give an overall inter-particle attraction. Here the particles stick together on sedimentation, rather like toffee coated ball bearings. This results in a random, close-packed sediment.
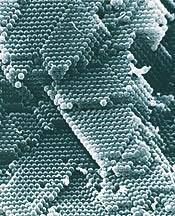
While the first example represents a system that is colloidally stable, it is not always desirable. Particles bigger than about 0.1?m in diameter will sediment, in time, depending on their density. If the particles are repulsive, a tightly packed, shear thickening sediment is formed, which can be difficult to re-disperse. This can happen in small pots of model paint; if left unused for a few months the pigment that sediments requires significant stirring or shaking to re-disperse.
Transporting slurries of ~30 per cent solid content on a 1t scale represents a more difficult problem. This can be overcome by introducing a weak attraction between the particles, resulting in a loose-packed sediment as shown in the second example. Here, owing to the more open network, the sediment is shear thinning and readily flows or re-disperses. The label ’Shake thoroughly before use’ is often stated on domestic products of this type. Manipulating the attractive-repulsive forces in dispersions is difficult, requiring a thorough appreciation of colloidal forces.
An analogy to the problems of a close-packed sediment can be appreciated by observing the area around one’s own footsteps when walking on the wet sand of a beach. The sand particles are densely packed. The shearing action of our foot causes the layers of sand particles to move up and over the lower layers. When this happens, the interstitial or void spaces between the particles increase in size and then take in more water resulting in a dry appearance at the top surface.
If a visit to the beach is inconvenient, try the following simple experiment. Weigh out approximately 63g of domestic cornflour into a cup. Add approximately 50cm3 water. Use a teaspoon to mix the cornflour and water very slowly. After a couple of minutes, a creamy mixture will form. Observe the difference between stirring with the teaspoon very slowly and quickly. Slow stirring allows the mixture to flow while rapid stirring is impossible. Allow the teaspoon to settle to the bottom. Pull quickly on the teaspoon and the cup will lift.
Mike Garvey
Making an Impact
The Impact Faraday Partnership was established in December 2000 and is helping to coordinate colloid research between industry and the academic base. The focus of Impact is to support the design and development of innovative materials, and the development of new formulations, across all sectors of industry including:
| Paints and coatings | Agrochemicals |
| Pharmaceuticals | Personal hygiene |
| Soaps and detergents | Petrochemicals |
| Specialised organics/inorganics | Foodstuffs |
Impact has three hub partners:
- Bristol Colloid Centre (BCC)
Established in October 1993, the Bristol Colloid Centre is a consultancy group that is strongly linked to the School of Chemistry at the University of Bristol. - Campden & Chorleywood Food Research Association (CCFRA)
CCFRA Group is the UK’s largest independent membership-based organisation carrying out R&D for the food and drink industry worldwide. - Institute of Applied Catalysis (iAc)
The Institute of Applied Catalysis is an independent, knowledge-based, industrially led virtual institute dedicated to promoting the application of catalysis through its education, networking, and research programmes.
Impact’s objectives are:
- To facilitate interactions between companies and universities to make advances in meeting the scientific engineering and technological challenges in the broad area of colloids and interfaces.
- To generate a strategic research plan for the industrial and academic colloid community based on technological needs and capabilities and research challenges.
- To create a mechanism for developing step-change industry-led research activity in colloids and interfaces.
- To design and develop a modular, distance-learning programme in colloid technology, for use by industrially based and academically based personnel.
- To sponsor networking activity aimed at encouraging the dissemination of scientific and technological knowledge.
To facilitate the above, Impact has in place five Technology Translators. They are the key personnel associated with recognising and communicating the available technological capability and brokering partnerships between members of the industrial base and scientists and engineers.
For further information about Impact or your local Technology Translator visit impactfp website or contact the Impact office.
Effective delivery
Already used in a wide array of conventional pharmaceutical formulations, colloids underpin many emerging drug delivery technologies. By manipulating physical characteristics, such as size, it is possible to control the biological fate of particles in the body, and thereby deliver drugs more efficiently to their sites of action.
Aerosols of microparticulate drugs are widely used to treat pulmonary diseases, such as asthma. The formulations themselves usually consist of drug particles dispersed in volatile propellants (metered dose inhalers) or blended with a carrier such as lactose (dry powder inhalers). The particle size of the aerosols these devices produce then governs their site of deposition. Too large (>10?m) and the particles’ inertia causes them to impact on the patient’s mouth or pharynx, rather than reaching their intended target - the central airways of the lung. Too small (<2?m) and the particles may penetrate the more peripheral airways (alveoli), with subsequent absorption into the blood circulatory system.
Reducing particles to the ?m size range (micronisation) is commonly used to increase the surface area of drugs and thereby enhance their dissolution rate. However, in order to increase the saturation solubility of a poorly soluble drug, to achieve sufficient levels in the blood after oral administration (bioavailability), the micronised powder must be reduced to nanoparticles. Reducing the particle diameter below approximately 1?m increases the number of drug molecules dissolving relative to the number of molecules recrystallising on the particle surface. The increase in solubility that this produces can often dramatically improve oral bioavailability. Nanoparticle technology can also be combined with traditional dosage forms by, for example, incorporating the drug nanoparticles into tablets.
Often, the drug particles themselves are unstable physicochemically and/or biochemically in harsh biological environments, such as gastric juice (low pH) or in the presence of blood proteins. In such cases, one option is to encapsulate the drugs in polymeric particles, assembled from biodegradable polymers such as polylactic acid. A carrier particle expands the range of options for tailoring the particle properties to achieve delivery to specific sites. In particular, block copolymers can be used to form particles with a core-shell architecture, with the hydrophobic core acting as a reservoir for a wide variety of drugs.
Hydrophilic polymers, including polyethylene glycol, are commonly used to provide the outer shell of these systems. In the aqueous medium of the bloodstream, these hydrophilic chains form a dense steric barrier that can allow the particle to evade capture by cells (phagocytes) of the body’s natural defence system. Consequently, the particles can achieve prolonged blood circulation times and redirection to other organs and tissues. Unfortunately, difficulties in producing polymeric nanoparticles on a large scale have hampered their emergence in marketed pharmaceutical products. Nevertheless, given their ability to achieve targeted delivery, their widespread use particularly for the delivery of genes and anticancer agents is expected.
Trevor Riley, GlaxoSmithKline R&D
Colloid rheology
The way that toothpaste squeezes from its tube, the consistency of shower gels and the mouth-feel of yogurt and fruit juice are all controlled by their rheology - how these everyday colloidal products flow and deform when in use. Rheology plays a major role in manufacturing these products well before they reach supermarket shelves. The rheology of these colloids must be optimum during mixing, stirring, pumping, coating, spraying or extrusion processes. It follows that chemists and technologists working in areas as diverse as paints, inks, foods, agricultural chemicals, pharmaceuticals, electronics and petroleum recovery need to understand the relationship between the rheology of their products and their composition. In turn, the flow behaviour of these products is controlled by their microstructure - the way in which the constituent colloidal particles interact and self-assemble.
A rheological experiment - such as measuring the viscosity of a material - helps us to understand the relationship between the microstructure of a material and its composition. If we also know how, for example, the viscosity of a material influences its behaviour during manufacture or use, we have a powerful tool that can help us to make better products or understand when things go wrong. Careful formulation is the way to avoid problems such as slimy and stringy shower gel or all the chocolate solids settling in milkshake.
Rheologists are interested in the relationships between stresses and strains in materials. Stress is the force acting on a material per unit area, as it is stirred or stretched. Strain is simply the increase in length of a material, compared with its original length; think of the Hooke’s Law experiment, when the length of a wire is increased as weights are suspended from it. As well as stretching a material, another important effect is ’shearing’. Material is sheared when it is confined in a layer between a fixed surface and a moving surface. Under shear layers of the material slip past each other, a bit like a pack of playing cards being spread out. In liquids, the rate of change of strain with time - the shear rate - is important. Viscosity is simply the stress divided by the shear rate.
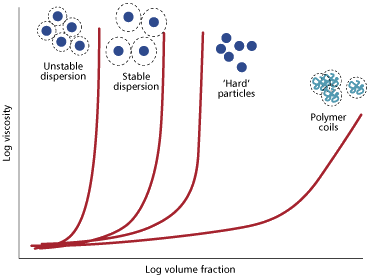
In considering the rheology of colloidal systems, the formulation chemist must understand how the viscosity increases with particle concentration. The simplest example concerns hard spherical particles that don’t interact, ie there are no forces acting between them. At low concentrations there is enough liquid between the particles for the material to flow like a liquid. At high concentrations, the particles take up more volume and bump into each other, causing an increase in viscosity. At very high particle loadings the particles touch and the system behaves as a solid. Attraction between these particles can be overcome by making the particles charged, or ’hairy’ by adsorbing a polymer stabiliser on their surface. The presence of a ’stabilising layer’ increases the effective volume of the particles so tends to give a higher viscosity than the ideal system. Rod-like particles don’t pack as effectively as spheres and tumble as the material flows, so again give a higher viscosity for the same volume fraction.
Unstable particles that attract each other form clusters that trap the solvent. This again increases the volume taken up by the particles and increases the viscosity. As the number of unstable particles is increased, the clusters link together to form a continuous network. This is what rheologists call a gel. Gels behave more like solids and have a very high viscosity.
Rob English, North East Wales Institute of Higher Education






No comments yet