How Harry Lochte (1892–1976) spun up a separation revolution
Distillation is typically a slow and static affair. As Primo Levi put it in his Periodic Table: ‘I love distillation because it is slow philosophical work … that gives you time to think, a bit like riding a bicycle.’ If the connection seems tenuous, in the 1930s there was a convergence between the seeming stillness of lab distillation and the whirling frenzy of a moving bike.
At the start of the 20th century, the growth of the oil refining industry created demand for methods to deal with the mind-bogglingly complex mixtures emerging from oil fields. Contemporaneously, Francis Aston’s discovery of isotopes presented the challenge of separating chemical species that barely differed from one another.
While empirical designs for static fractionators were reported in their dozens, theory lagged behind experiment. Ernest Sorel was the first to have taken seriously the idea of an equilibrium between material in the vapour and in the condensed phase. He introduced the idea of the theoretical plate, which led him to an effective, but very tedious, mathematical description of the process. His German contemporary Eugen Hausbrand developed a parallel method that focused more on the thermodynamics of the process, considering the energy changes that accompanied vaporisation and condensation.
Warren Lewis at the Massachusetts Institute of Technology in the US was a key figure in developing Hausbrand’s and Sorel’s work into a predictive theory of distillation; by the 1920s a number of useful simplifications and short-cuts to the mathematics had been discovered. William Peters at DuPont proposed using the height equivalent to a theoretical plate (HETP), today a key figure of merit for a distillation device, while Warren McCabe and Ernest Thiele devised a graphical method for making predictions without having to wade through pages of calculations.
At the heart of everything were considerations of mass transport – how fast could molecules be moved around in the fractionator itself, as well as from the liquid to the vapour and back.
Spinning out
In 1936, the physicist George Pegram proposed a wholly new form of fractionator while collaborating with Harold Urey on the separation of oxygen isotopes. It consisted of a rapidly rotating central shaft bearing a series of equally spaced metal cones, between which would sit a corresponding number of stationary counterparts. Liquid condensing on a spinning cone would be flung to the wall to drain back towards the shaft and onto the next moving cone. With a column ‘not prohibitively long’ (35 feet tall with 619 pairs of cones) it was possible to get a two-fold enrichment of 16O and 18O.
It didn’t catch on. But the idea of a spinning core may have planted a seed. Harry Lochte was descended from German settlers in the US who eventually established themselves in the fertile plains around Fredericksburg, Texas. H L, as he was known, showed an academic bent from an early age. While his brothers were farmers, H L started teaching at local schools and then studied chemistry at the University of Texas at Austin. His graduate work was interrupted by the first world war but he later finished his PhD with William Noyes at the University of Illinois Urbana–Champaign.
He returned to a faculty position at Austin in 1922, teaching organic chemistry and working, of course, in petroleum chemistry. He was made responsible for the library and played a key role in saving it in 1926 when the building caught fire, protecting the books with wet tarpaulins while the firemen battled the blaze. By 1927 he had become the chair of his department, overseeing the teaching of organic chemistry and characterising the peculiar molecules found in oil shales and petroleum.
In 1937 he was joined in the lab by a mature student, another ex-teacher, Sherman Lesesne. Also from Texas, Lesesne studied chemistry at a teacher training college in Huntsville, graduating with a BSc in 1931. He obtained a master’s degree in 1932 from Southwestern University and stayed on to teach there and in a local school until he joined Lochte.
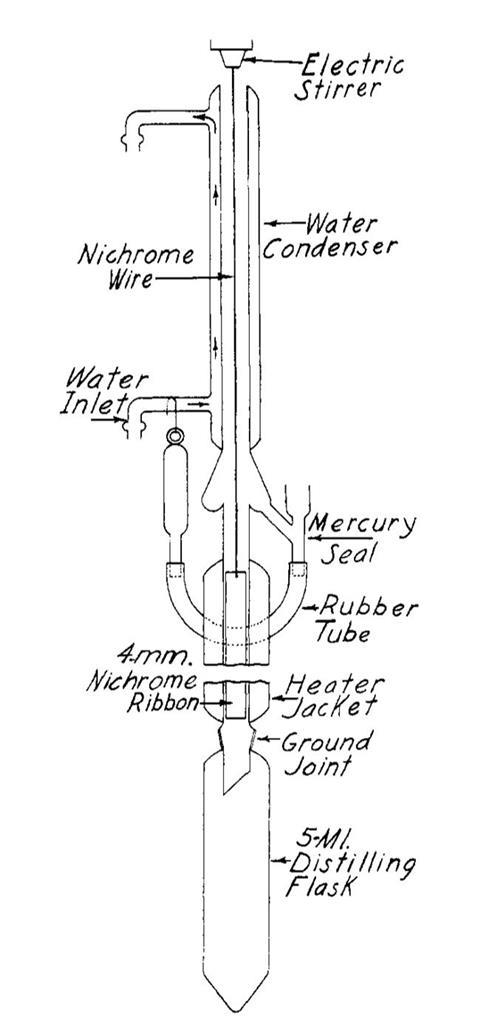
It is not clear how the idea for the fractionator came about; the paper they published in 1938 only refers to static distillation devices. But their idea was different. The fractionator, which Lochte well have assembled using the gas torch in the lab next to his office, consisted of a hollow tube in the centre of which sat a close-fitting sheet of metal. During distillation this band revolved at 1000rpm, causing any liquid condensing on the surface to be spun into a film and then flung onto the walls of the tube. The wind of vapour inside the column sped evaporation of the more volatile components out of the liquid at any height. The performance of the column was impressive.
Neither Lochte nor Lesesne followed this up. After doing a PhD on the chemistry of quinolines with another academic, Lesesne may have gone back to teaching, but all trace of him is lost aside from his death in 1994. An easy-going person, popular with students, Lochte would continue at Austin until his retirement in 1967.
In the decade after their report, variants of their fractionator, typically with longer, twisted ribbons, were reported for both atmospheric and reduced pressure distillation, their advantage being their very low liquid holdup. I have never used one, but I remember two mysterious, mothballed stills sitting in a corner of our fourth floor for years. They are now long gone. I would love to see one in action and to meditate philosophically on the meaning of chemistry and the passage of time.
References
S D Lesesne and H L Lochte, Ind. Eng. Chem., Anal. Ed., 1938, 10, 450 (DOI: 10.1021/ac50124a023)
















No comments yet