From the Olympics to New Year's Eve events, fireworks are synonymous with celebration. James Mitchell Crow looks into some pyrotechnic research worth celebrating in itself
From the Olympics to New Year’s Eve events, fireworks are synonymous with celebration. James Mitchell Crow looks into some pyrotechnic research worth celebrating in itself
Bangs and crashes, bursts of light, flashes of colour - few can resist the delights of a fireworks display. Pyrotechnics like those that light up our night skies on New Year’s Eve have been enchanting young and old for almost 2500 years, since the Chinese first discovered that when organic matter is mixed with potassium nitrate, an oxidiser, it burns with an impressive flash and bang. Over the millennia, fireworks have slowly evolved. Yet despite this long history of development, fireworks formulations could be about to undergo one of the biggest shifts in their history.

Today’s fireworks are not the most environmentally friendly of entertainments, containing toxic components that can range from perchlorates to heavy metals. Non-toxic replacements for these compounds, and many other fireworks ingredients besides, are beginning to emerge from the chemistry lab.1
Send in the army
While the environment would undoubtedly benefit from cleaner civilian fireworks displays, it is the military that is really driving forward this research area. The armed forces are particularly heavy users of pyrotechnics, from simulated explosions on the training ground to the signal flares, illuminations, decoys and smoke screens used on the battlefield. Within the military there is a growing realisation of the potential health impact on troops of continual exposure to the combustion products of pyrotechnics, as well as the environmental impact.
’In the past decade, the environmental movement has really spread to the military,’ says Jesse Sabatini, a research chemist working for the US Army to clean up pyrotechnics formulations. ’In the old days of military research, the main thing was just to get something out to the field; there wasn’t really much concern for the environmental aspect. And now there is - the US Department of Defense is now paying lots of environmental clean-up costs. It would make sense, going forward, that we do develop something sustainable.’

’Research in this area is growing,’ agrees Thomas Klap?tke, a chemist researching explosives and pyrotechnics at the Ludwig-Maximilian University of Munich (LMU) in Germany. ’There is money available, not just for work to improve pyrotechnic performance, but also for improving their environmental impact and sustainability.’
Green pyrotechnics research is being driven forward by the US, explains Klap?tke - largely thanks to the country’s combination of strict environmental controls and its strong tradition of armed forces R&D. ’The whole area of military research is bigger in the US,’ he adds.
While the armed forces might be the first to benefit from this work, military-funded research into cleaner pyrotechnics is already starting to filter through to civilian fireworks displays, and the potential for growth is huge (see box).
Quit smoking
Until recently, the basic components of a conventional firework hadn’t changed much since ancient times. A carbon-based fuel - the reducing agent - is mixed with a metal salt that acts as both the oxidant and the flame colourant. In more modern formulations, powders of metals such as magnesium are used as the fuel, resulting in a brighter burning firework. However, the underlying energetics remain the same - lighting the firework starts a highly exothermic process as the fuel rapidly reacts to form oxides.
When watching a fireworks display, perhaps the most obvious pollutant that results from this process is the smoke. Smoke is a by-product of the incomplete combustion of carbon-based components, leaving behind inhalable particulate matter with various health implications. Smoke is also produced by the combustion of metal fuels, which generate tiny metal oxide particles. As a result, smoke is essentially an unavoidable by-product of conventional firework chemistry.
Around a decade ago, however, a team of researchers at Los Alamos National Laboratory (LANL) in New Mexico, US, began to work on pyrotechnics based on a very different kind of chemistry. Nitrogen-rich compounds also pack an energetic punch when they burn, not through the production of oxides but because of the large amounts of energy released when these compounds break down to release nitrogen gas.
’Our original work was published in the late 1990s, and it helped to spur quite a bit of interest in this area,’ recalls chemist David Chavez, one of the original LANL team.2 The group had previously been working on high-nitrogen compounds as explosives - but the low smoke levels that they observed suggested that fireworks might also benefit. So the team began to work on compounds with the lower level of kick needed for pyrotechnics applications. Compounds based on tetrazoles - five-membered rings with four nitrogen atoms and one carbon - have proven particularly useful.
For the military, the benefits of cutting smoke extend beyond health and environmental concerns, adds Klap?tke, who also moved into the pyrotechnics area after working on nitrogen-rich explosives. ’We are working on very-high-nitrogen caesium-containing flares for near infrared night time battlefield illumination,’ he says. ’People can’t see near infrared light, but that’s the good news - you can equip your own personnel with night-vision equipment exactly tuned to the wavelength of your flare, so you can see but the other side can’t. High-nitrogen compounds are really good in this area because they produce much less smoke.’ The result is a much brighter luminous intensity, and a battlefield that appears better lit to your own troops.
For the same reason, the lack of smoke in a high-nitrogen firework also means that less metal colourant is needed to achieve the same brightness. When the metal in question is barium, the heavy metal used for green-light-emitting fireworks, that’s a significant bonus. Chavez and his colleagues have developed bistetrazole-based compounds able to complex the metal in question, thereby finely dispersing the metal throughout the pyrotechnic formulation and so decreasing the amount of metal required still further.3
Happily ever after
At first glance, a Disney theme park might not appear to have much in common with a military training ground. But it turns out that both are amongst the heaviest users of pyrotechnics in the US, and their neighbours don’t like the resulting impact on their environment.
For Disney, the problem lay in the plumes of smoke that would drift out of the park and across surrounding suburbs following their frequent fireworks displays. Smoke isn’t just a problem with civilian fireworks displays - it is also a problem for military pyrotechnics, particularly for light-emitting flares, where the smoke can obscure the light.

In the late 1990s, that was just the issue that a team of chemists at Los Alamos National Laboratory (LANL) in New Mexico were trying to address using energetic compounds rich in nitrogen. ’During that time, Disney was interested in reducing the smoke in their outdoor pyrotechnic displays, and so they approached the group and asked what might be some possible solutions to their problems,’ recalls David Chavez, a member of the team who still works at LANL.
Disney’s fireworks are now based on derivatives of LANL’s original high-nitrogen compounds. A small business called DMD systems was set up by some of Chavez’s former LANL colleagues to develop Disney’s formulations. The commercial applications of the LANL research haven’t been limited to theme park displays, however. Since the early Disney research, the company has gone on to pioneer the use of high-nitrogen compounds for indoor pyrotechnics, used for example in concerts and theatres.
Cleaner colour
Chavez might have shown a way to reduce the amount of barium in a firework but last year, Sabatini and his colleagues at the US Army’s Picatinny Arsenal in New Jersey managed to replace the barium altogether - using a rather unlikely molecule.4
Sabatini’s barium replacement is based on boron. The team started by looking to replace barium in the US Army’s green hand-held signal named the M125A1. Previous research had indicated that a mixture of amorphous boron and potassium nitrate will generate the green-light-emitting boron oxide during combustion - but that it burns far too quickly for practical use. So Sabatini was looking for a low cost barium compound with a longer burn time.
The compound that the team came up with, boron carbide, is so unreactive at low temperatures that it is currently used as an industrial abrasive - not a promising property for a firework component. However, Sabatini came upon research from the 1960s showing that, at elevated temperatures, boron carbide will react with oxygen to give boron oxide, the desired green light emitter.
Sure enough, a mixture of potassium nitrate oxidiser and boron carbide fuel, held together with a polymer binder, gave a long-lasting bright green pyrotechnic. Having mastered the formulation in the lab, the team currently fine-tuning it so that its performance holds up when integrated into the green-light-emitting hand-held signal. ’We’re a couple of seconds away in terms of burn time, but our luminous intensity - how bright these things burn - is off the charts; it’s exceeding the military specifications by five or six times,’ Sabatini says. ’It not only has these military implications, I think it also has massive commercial potential,’ he adds.
The other challenge that the team is currently addressing is trying to understand just how boron carbide generates boron oxide in the first place, given that boron carbide is essentially inert at lower temperatures. ’Right now we are investigating the mechanism through which boron carbide gives you green light,’ says Sabatini. ’It’s one thing to put it in a formulation, and quite another to actually evaluate what the heck is going on, when on the surface the compound looks like it doesn’t do anything.’
In the meantime, the research to improve the basic formulation continues, in collaboration with Klap?tke and his colleagues at LMU. Sabatini’s prototypes employ potassium nitrate as the oxidiser, and it is the potassium that the team hopes to remove. Not because the metal is toxic, but because potassium is a red light emitter, which reduces the colour purity of the flare - making it harder to tell at long distances whether the flare is red or green.
’We have developed a boron-containing oxidiser,’ Klap?tke says. ’We are looking into coming up with a totally metal-free formulation only containing boron colourant and boron oxidiser - that will be the next step.
’If we manage to do this, we would increase the colour purity and also increase the intensity of the flare,’ Klap?tke adds. ’We can then either make the flare smaller, or make it burn longer and brighter - which would be advantageous to the war fighter for obvious reasons.’
The big smoke
For most pyrotechnics applications, smoky formulations are a curse, releasing particulate pollution that partly obscures a firework’s brilliant burst of coloured light. But for several military applications, from smoke-based signal flares to smoke screens, the opposite is true, and generating smoke is the main aim of the device.
That’s not to say that the smoke can’t be cleaned up. Reducing the toxicity of one of the US military’s smoke flares, the M194 yellow smoke flare, is one of the research objectives of chemist Jared Moretti at the US Army’s Picatinny Arsenal in New Jersey. ’The problem with the yellow smoke composition in that item lies in the toxic yellow dyes, particularly Vat Yellow 4 and benzanthrone,’ says Moretti. ’Both of those compounds contain the anthroquinone functional group, which is notoriously carcinogenic. We initiated a research programme to reformulate this composition to something more environmentally benign.’

As it happened, the US Army already had a less harmful yellow dye on its books, a compound used in smoke-releasing hand grenades known as Solvent Yellow 33, which relies on the much less harmful quinoline group for its yellow colour.
The challenge in developing effective military smoke-releasing flares is to produce items with a sufficiently short burn time, producing high volumes of smoke for 15 seconds or less - but that don’t burn so hot that they decompose the dye. Moretti and his colleagues are currently working on short-burning formulations using Solvent Yellow 33, employing compounds such as sodium bicarbonate in the formulation that cool the pyrotechnic by releasing carbon dioxide and water.
Cutting perchlorate
Perhaps the biggest targets for removal from fireworks formulations, however, are the perchlorate salts. Perchlorate (ClO4-)was once considered the ultimate oxidant for applications ranging from rocket fuels to fireworks, thanks to its stability, low cost, and particularly its large positive oxygen balance. When it burns, it releases four oxygen atoms from every molecule of perchlorate. ’Performance-wise, it is really very difficult to find something as good as perchlorate,’ Klap?tke says.
However, the environmental concerns around perchlorate are mounting, to the point where the compounds are increasingly regulated. Perchlorates are highly water soluble, and so can accumulate in groundwater, affecting drinking water supplies. The ion has been linked with birth defects, and can also impact thyroid function. In the US, the Environmental Protection Agency’s particularly tight legal limits for perchlorate levels in groundwater have severely limited the use of pyrotechnics on many US military training grounds.
’Many training camps have been in danger of having to close because of perchlorate groundwater contamination in towns and lakes nearby,’ explains Jared Moretti, a research chemist at the US Army’s Picatinny Arsenal. ’This is one of the major drivers of research in this area.’
Despite the challenges, perchlorate-free pyrotechnic formulations are beginning to emerge. Key to this process has been the emergence of high-nitrogen compounds as fuels. ’By using high-nitrogen compounds, you can dramatically reduce the amount of oxygen that you need,’ says Chavez. High-nitrogen compounds produce far fewer oxides than conventional fuels during combustion, and so oxidisers with a lower oxygen balance, such as nitrates, can replace the perchlorate with no loss of performance.
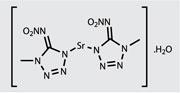
In fact, Sabatini and his colleagues have recently developed a high performing perchlorate-free red-light-emitting flare, thanks to the addition of a tetrazole-based high-nitrogen strontium salt.5 ’In addition to giving you more energy, the gases that the high-nitrogen compounds produce tend to expand the flame - they make the fireball bigger - and that is perceived by the viewer as the firework burning brighter,’ Sabatini says. The team were able to produce a perchlorate-free flare, which burns longer and brighter than the formulation that it replaces.
Again, the potential for such formulations to be adopted for civilian fireworks is clear. ’The driving force at the moment comes from the military, but once we have available nice green and red smokeless, non-toxic, heavy metal-free flares, I think they may very well find their way into fireworks,’ Klap?tke says.
James Mitchell Crow is a science writer based in Melbourne, Australia
References
1 G Steinhauser and T M Klapötke, Angew. Chem., Int. Ed., 2008, 47, 3330 (DOI: 10.1002/anie.200704510)
2 D E Chavez and M A Hiskey, JPyro, 1998, 7, 11
3 D E Chavez, M A Hiskey and D L Naud, JPyro, 1999, 10,17
4 J J Sabatini, J C Poret and R N Broad, Angew. Chem., Int. Ed., 2011, 50, 4624 (DOI: 10.1002/anie.201007827)
5 J Sabatini et al, Chem. Eur. J., 2011, DOI: 10.1002/chem.201102485






No comments yet