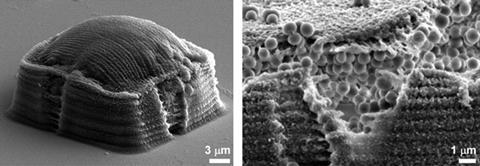
To build the prison, a laser is used to initiate a radical reaction where a photosensitiser, methylene blue, cross-links protein monomers together to form a hydrogel structure. The cross-linking only occurs close to the focus of the laser beam, so the researchers can build up the walls by raising the focus step-by-step, until the walls are about 10µm high. A roof is then created by scanning the laser across the top of the walls, trapping whatever is inside in a 6250µm3 size prison cell (video).
Current techniques for studying single cells rely on microfluidics, where cells suspended in a solution are passed through micro-scale paths and barriers which are designed to trap cells. ‘The real uniqueness about [our] approach is it’s targeted isolation of single cells, unlike all the competing approaches ... where you might stochastically trap a cell,’ Kaehr explains. ‘Here, if we have a rare environmental cell that may show up one in a million, we can screen for that and target that specific cell in one of these chambers and then do single cell chemistry or analysis.’
The chamber is constructed in such a way that nutrients and waste products can diffuse in and out, but other cells can’t enter. As a result, the trapped cell is free to replicate without interference from other cells. Within a few days, a cell can replicate so many times that the chamber fills and the roof bulges outwards.
The team tested this technique on Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria, as well as yeast (Saccharomyces cerevisae). With the current setup, only one in three cells that are isolated can survive and reproduce. This could be due to several factors, but the main issue is the formation of toxic reactive oxygen species produced by the radical reaction.
However, David Weitz, of Harvard University, US, says: ‘When I read it, I just thought “It’s beautiful, it’s lovely, but what would I do with it?” and I haven’t got an answer.’ Kaehr has a few ideas, including studying cell signalling, but is still on the lookout for other applications. ‘I’m in the game of trying to develop techniques for biology. This is a nice technique where we can shop around and see what are the interesting problems that biologists have.’






No comments yet