Japanese researchers have used atomic force microscopy to film the enzyme that synthesises ATP
Japanese researchers have used a high-speed atomic force microscopy (AFM) to shoot a movie of the tiny rotating enzyme that produces the chemical fuel for cells. There are plenty of still pictures of ATPase, the enzyme that synthesises adenosine triphosphate (ATP), thanks to X-ray crystallography and pulsed-laser methods. However, ’moving’ pictures should offer scientists a much clearer view of the enzyme’s inner workings.
ATPase contains three subunits that generate rotational force in one direction, torque, by sequentially changing from closed to open conformations, rotating the enzyme’s central shaft in 120? steps. The rotation converts an electrochemical stimulus - the movement of protons - into chemical energy. In reverse, the rotation driven by ATP hydrolysis produces an electrochemical output.
The enzyme has two rotary components, F0 and F1; the former is a proton-flux driven motor embedded in a membrane. The latter is the water-soluble, ATP-driven motor. They are connected through the common central shaft and the peripheral stator-stalk that anchors the stator parts of the two motors. The rotation of the F1 component fuelled by ATP was first videoed by Hiroyuki Noji of the University of Tokyo in 1997 and its rotary dynamics have been well studied. However, no one has yet fully explained the structural basis of its cooperative torque generation.
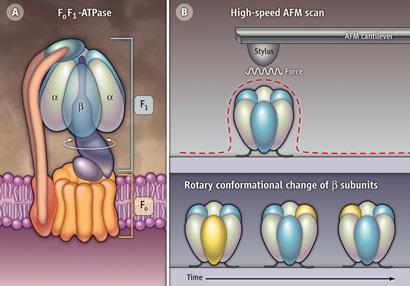
Now, Noji and colleagues have used AFM to record a sequence of frames of the rotorless F1 component (the catalytic stator ring). When fuelled with ATP, the three beta subunits move through their closed and open conformational states in an anticlockwise direction. The team explains that even in the presence of the ATP analogue, adenylyl imidodiphosphonate, only two beta units can assume the closed conformation at the same time ensuring that one is always open and so ready to cycle. This, the team suggests, implies that the direction of rotation is programmed into the stator ring, rather than the shaft, which thus dictates the direction of drive.
The findings further improve our understanding of the enzyme, which was highlighted in the 1997 Nobel Prize in Chemistry, awarded in part for the elucidation of the enzymatic mechanism underlying the synthesis of ATP. Noji’s work also has implications for our understanding of the structurally related hexameric enzymes involved in the degradation of proteins and the unwinding of DNA and RNA.
’This work clearly indicates that it is possible to observe structural changes of the ATPase motor proteins using AFM,’ says Cindy Berrie of Kansas University. ’It has the advantage that it can be carried out in physiological conditions without labelling of the protein.’ She adds: ’The observation that conformational changes occur without the presence of the gamma subunit will prompt more investigations of the possibility of cooperativity in the ring itself. While this does not settle the issue of the mechanism of torque generation in the F1-ATPase molecular motor, it provides valuable new insight into this critical question.’
David Bradley
References
T Uchihashi et al, Science, 2011, 333, 755 (DOI: 10.1126/science.1205510)






No comments yet