Researchers create a recyclable membrane based on non-covalent bonds to filter nanoparticles
Israeli researchers have created a recyclable membrane based on supramolecular linkages that can be used to filter nanoparticles. The membrane, which unusually comprises non-covalent bonds, performs just as well as conventional sieves, offering a green and versatile alternative for size-separation and purification of nanoparticles.
Standard filtration membranes are usually held together by strong covalent bonds, which give membranes suitable strength to withstand the pressures involved in filtration processes. The problem is that when membranes become clogged up they have to be discarded and replaced.
One idea is to make membranes based on supramolecular (non-covalent) interactions which can undergo reversible self-assembly. Since the bonds can be undone easily, they offer a recyclable and adaptable option. But making such membranes with a level of robustness to rival conventional options has remained a challenge.
Now, Boris Rybtchinski and colleagues at the Weizmann Institute of Science in Rehovot, Israel, have managed to make a robust and recyclable untrafiltration membrane with non-covalent hydrophobic linkages. ’This results in easy fabrication, recyclability, and versatility that cannot be achieved with regular covalent materials,’ says Rybtchinski.
The team created a compound that self-assembles in water. It has a specially designed large and flat hydrophobic surface. ’In water, these surfaces experience very large attractive forces that hold them together, eventually forming porous nanostructured 3D networks possessing high robustness,’ Rybtchinski explains. By filtering these structures onto a cheap commercial support with 400nm pores, they form a nanostructured membrane that works as a nanoparticle sieve.
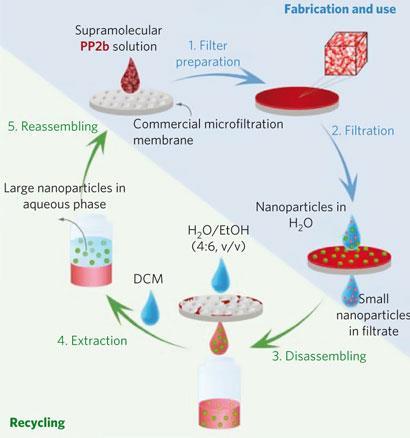
The hydrophobic interactions are strong enough to hold together the membrane and withstand the flow of particles, says Rybtchinski. Experiments with solutions containing gold nanoparticles of various sizes revealed that only particles smaller than 5nm could pass through a 12?m thick membrane. By increasing the thickness to 45?m, the team discovered that the membrane could separate smaller particles (CdTe quantum dots of 2-4nm in size) because of a time delay between different sized particles passing through the membrane, resulting in size-selective chromatography.
The membrane is easily disassembled by adding solvents such as ethanol which weakens the hydrophobic interactions. ’This way the material can be retrieved, cleaned, and reused for fabrication of another membrane,’ says Rybtchinski. Furthermore, particles that are stuck in the filter can be recycled too, which is not always possible with conventional membranes.
Jonathan Nitschke, who researches self-assembling polymers at the University of Cambridge, UK says that Rybtchinski’s use of non-covalent interactions to knit together a filtration membrane is innovative. ’Supramolecular linkages can be undone under certain conditions, allowing the membranes to be dissolved and recreated so it’s an excellent way of cleaning and recycling them.’
James Urquhart
References
E Krieg et al, Nature Nanotechnology, 2011, DOI: 10.1038/nnano.2010.274






No comments yet