Researchers boost the number of proteins that can be held inside the empty shell of a virus, bringing nanoreactors a step closer
Scientists in the Netherlands have devised a way to pack large numbers of proteins into the empty shell of a virus. The technique opens the way to creating a nanoreactor containing multiple enzymes or mimicking cell organelles, where proteins and metabolites exist in a tightly confined space.
For many years the cowpea chlorotic mottle virus (CCMV) has been used to generate a nanometre-sized capsule for carrying ’guest’ molecules. If the virus is held at pH 7.5 its outer protein shell or capsid disintegrates. The RNA contained within the capsid can then be removed and the capsid reassembled simply by lowering the pH to 5.0. If the capsid is rebuilt in the presence of other molecules, such as proteins, it will capture a proportion of these within it.
Using this approach, the capsid will typically encapsulate only one or two guest protein molecules, but Jeroen Cornelissen and his team at Radboud University Nijmegen have now shown it is possible to trap more proteins within the shell.
The researchers attached the guest molecule to be encapsulated to individual fragments of the capsid in its disassembled state. When the capsid reassembled, it would enclose the new molecules that were stuck to the building blocks. Importantly, the guest molecules would need to be held in place by non-covalent bonds: earlier studies indicated that a covalent bond locks the guest protein too rigidly in position and can interfere with its activity and with reassembly of the virus.
Instead, Cornelissen and his team constructed an anchoring system that was sufficiently strong to keep the guest protein attached to the capsid fragment, but weak enough to allow the capsid to reassemble and the protein to function normally once inside.
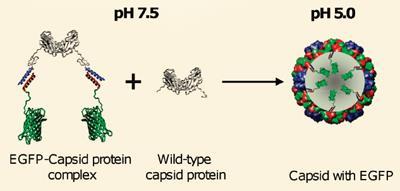
They did this by generating a genetically modified version of the capsid protein fragment possessing a coiled amino acid ’tail’ with particular hydrophobic and charged portions. A ’complementary’ tail can be similarly engineered onto any given potential guest protein. In solution the two tails intertwine and become clamped together through hydrophobic and electrostatic interactions. In this way the guest protein is incorporated into the reassembled capsid.
’We used green fluorescent protein as a model system and the maximum loading we can achieve is around 15 to 20 protein molecules per capsid,’ says Cornelissen. ’This is around a tenfold increase from the one or two molecules that are encapsulated normally.’
’For the first time the potential for inclusion of multiple enzymes in the confined space of a virus-based nanoreactor has been realised,’ says Dave Evans, an expert on viral capsids at the John Innes Centre in the UK. ’Excitingly, further development should lead to bionanoparticle-confined multi-enzyme cascade reactors and to model systems for the metabolic pathways of simple cell organelles.’
Simon Hadlington
References
I J Minten et al, J. Am. Chem. Soc., 2009, DOI: 10.1021/ja907843s






No comments yet