The exact crystal structure of the stable form of a widely used semiclathrate hydrate has finally been elucidated – 80 years after the material was first discovered. The work provides deeper understanding for researchers aiming to use these cage-like structures in various industrial applications.
Clathrate hydrates comprise frameworks of water molecules surrounding small, covalent guest molecules. They have applications such as gas storage and water purification. When larger, ionic molecules replace these non-polar guest molecules, they can displace some of the water molecules and insert into the cage structure to form semiclathrate hydrates. The best known and most widely used of these, with potential applications in heat storage and advanced air conditioning technology, is tetrabutylammonium bromide (TBAB) hydrate.
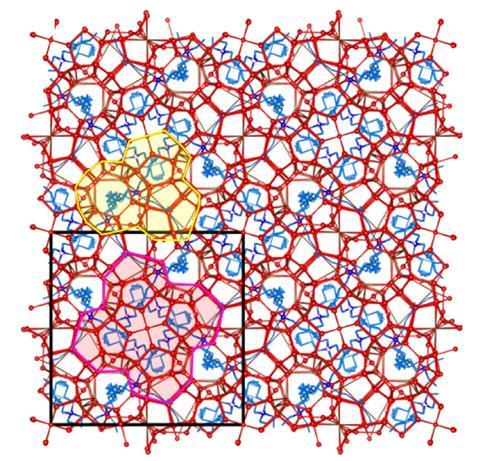
However, like some other semiclathrate hydrates, TBAB hydrate exhibits pseudopolymorphism – a type of structural variation in which the number of bound solvent molecules changes along with the crystal arrangement. When an aqueous solution is simply cooled under ambient conditions, two pseudopolymorphs form. The first, TBAB·38H2 O, is a metastable orthorhombic form, whereas the stable TBAB·26H2 O is tetragonal.
‘The molecules are large and the number of water molecules is large,’ explains physical chemist David Wu at Academia Sinica in Taipei, Taiwan, who was not involved in the new work. ‘You have competing structures that are possible and they just differ slightly in how preferred they are. In addition, even for a single pseudopolymorph, it can be hard to tell the difference between candidate structures.’ Despite this, researchers in Japan resolved the structure of the metastable form in 2005. However, 80 years after the molecule’s discovery, the exact structure of the stable form has remained a mystery.
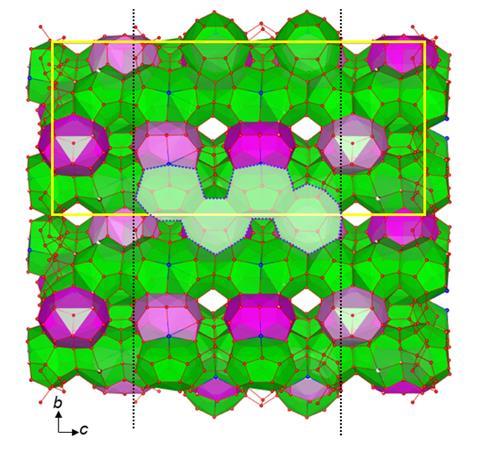
In the new research, a team led by Sanehiro Muromachi at Yokohama National University in Japan slowly grew perfect crystals of the material in the stable phase from solution before quenching them in liquid nitrogen. They then irradiated them at Japan’s Spring-8, the most powerful synchrotron beamline in the world. By interpreting the resulting diffraction patterns, the researchers determined that the structure contains features such as a single bromide ion taking the place of two water molecules – analogous to that seen previously in tetrabutylammonium nitrate. The researchers hope that this exact structure determination will optimise applications of this material and potentially allow for more rational design of new materials in future.
‘The principles elucidated in this study, particularly the ability of the TBA cation to adapt to primitive water clusters … extend beyond clathrate hydrates and provide insights applicable to the scalable design of related water-based functional materials, such as clathrates of group 14 elements, surfactants, and functional polymers,’ the researchers conclude.
‘This is an important structure to know, and they went through and were able to carefully resolve that question,’ says Wu. ‘It would be interesting to know whether these structural motifs show up in other semiclathrate materials.’
References
S Muromachi et al, Cryst. Growth Des., 2025, DOI: 10.1021/acs.cgd.5c00364














No comments yet