Readers muse on thermal hazard data, and ask for help with an embroidered periodic table
Periodic blackwork
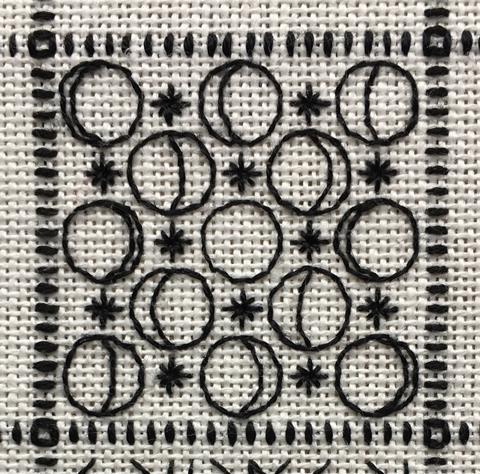
I would like to invite readers to contribute ideas for my project to create a periodic table in the form of a blackwork embroidery sampler. Each element is represented by a unique pattern – a visual clue to the element. My designs take their inspiration from the element’s name, its discoverer or location of discovery, its structure, properties or uses, or from its alchemical symbol or the symbol used by John Dalton. The element pictured is selenium, named for Selene, the Greek goddess of the moon.
The project was inspired by the International Year of the Periodic Table, and in particular by the discovery of an 1885 periodic table at the University of St Andrews and the exhibition at St Catharine’s College, Cambridge, which included Mendeleev’s original publication among other early forms. It struck me that while most modern periodic table representations use colour – for impact, information or both – it would be nice to take it back to its original monotone and to delve into the history of each element.
I have completed the p-block and am working my way back across the transition elements. There are several elements for which I am struggling for ideas, and I anticipate that the lanthanoids and actinoids will be even more challenging. I would love to hear any suggestions you may have.
The elements completed to date can be seen on periodically.blog with an explanation for each. I hope that you enjoy browsing them, perhaps learn a few interesting facts, and feel inspired to get involved. You can contact me by commenting on the blog posts, on Twitter (@clareewilkes) or by emailing periodically.blog@btinternet.com
Clare Wilkes
Cambridge, UK
Thermal hazards
The article about Leslie Bretherick prompted me to recall a serious explosion that happened many years ago. A large-scale preparation (13 moles) of 2-azidoethanol (CAS 1517-05-1) detonated, knocking a metre-wide hole in the concrete laboratory partition. The blast also brought down the ceiling and deposited window glass 50 yards across the road. Fortunately, no-one was hurt.1
Nowadays, such an incident should be unlikely to occur, because the evaluation of thermal hazards has become standard practice when preparing a risk assessment for a chemical reaction. As a first step, one would consult the material safety data sheets and a suitable reference for the various reactants and products, identify any thermally hazardous functional groups (in this case azide) and then proceed to obtain differential scanning calorimetry (DSC) data on a sample. Not everyone, of course, has access to the equipment necessary to obtain DSC data, thus the safety data sheet can provide an important warning concerning the thermal hazards, as well as exposure hazards of a compound. I was shocked to discover that several suppliers of 2-azidoethanol omit appropriate warnings regarding its thermal hazards in the safety data sheet.
I believe it is about time that commercial suppliers include DSC data for chemicals with the safety data sheet. Presumably these data have been generated during the development of the manufacturing process and consequently could easily be made available. It would be even more useful if there was an open-access database of DSC data, which could also include non-commercial compounds (an enhancement to ChemSpider?). However, DSC data provide a warning of potential thermal hazards for single compounds in a neat state; they are not a substitute for drop/fall hammer testing or more sophisticated hazard assessments, nor do they give information regarding reaction mixtures. It is true that DSC data require interpretation and can vary depending on the data measurement method and the degree of purity, but instruction is readily available.
In the interest of helping professional chemists to use potentially thermally hazardous reagents safely, I would urge manufacturers to share this data and update safety data sheets to reflect all available information. Academics who report new synthetic reagents should include DSC data, if appropriate, with their publication.
Jonathan Fray CChem MRSC
Canterbury, UK
Reference
1 I C Appleby, Chem. Ind., 1986, 10, 337
Corrections
The Trace Analysis article on wildlife crime (Chemistry World, October 2020, p71) stated that a law had come into effect in the UK to introduce an ivory ban; to clarify, while the act has been given Royal Assent, the law has not yet been implemented.
The Significant Figures article on Stephanie Horovitz (Chemistry World, November 2020, p36) should have mentioned that the opening quote (from a letter from Kasimir Fajans to Elizabeth Rona about Horovitz’s tragic death) was taken from Geoff and Marelene Rayner-Canham’s chapter Stefanie Horovitz: A Crucial Role in the Discovery of Isotopes in the book Women in Their Element, World Scientific 2019, pp296–297. We apologise for this omission.












No comments yet