A creative balance between fields

A mashup is the very essence of human creativity: taking existing thoughts, materials, tools and reassembling them in new ways. If the term most commonly describes musical invention, from JS Bach to the Chemical Brothers, it is also ever-present in science where working ‘between fields’ very often results in analogous innovation. A great example of this is the invention of the quartz crystal microbalance (QCM), a device of exquisite sensitivity that appeared out of the blue at the end of the 1950s from a young man with a big idea.
Günter Hans Sauerbrey was born in Berlin, the son of a civil engineer. His father Willy managed to avoid recruitment into the Wehrmacht during the Second World War by virtue of his profession. The family survived the war unscathed and Günter chose to study physics at the Technical University in Berlin (TUB), starting his studies in 1951. It seems that it was there that the germ of his idea first began to crystallise in his mind.
The piezoelectric effect had been discovered in 1880 by the Curie brothers, Pierre and Jacques, who showed that non-centrosymmetric crystals such as quartz, Rochelle salt or calcite developed opposite electrical charge on opposing surfaces when compressed. Its uncanny similarity to the known phenomenon of pyroelectricity (the appearance of charge at the surface of crystals when warmed or cooled) inspired Gabriel Lippmann to propose a law of conservation of charge. His elegant analysis led him to the conclusion that an inverse effect must be expected: a crystal in an electric field should deform. Within six months, the Curies had confirmed Lippmann’s surmise.
By the 1920s, quartz crystals were being made to oscillate in complex electronic circuits at frequencies determined by the thickness of the crystal and the specific mode of vibration chosen. The crystal radios that older readers may have built as children were based on just this principle: the oscillation could be used to control or tune the radio circuit. By the 1930s Louis Essen would use the exceptional reliability of these vibrations as the basis for improved standards of timekeeping, methods that persisted until the advent of atomic clocks.
But quartz oscillators weren’t perfect. Their frequency was affected by temperature, humidity and especially by contamination. It occurred to Sauerbrey that perhaps he could make a virtue of such ‘defects’ and use the change in vibrational frequency of a crystal to measure the mass of material deposited on its surface. Others had had similar ideas; a patent for a piezoelectric humidity monitor had been filed in 1945. Sauerbrey would go much further.
We will probably never know how he came to do his diploma work with Hans Boersch. The head of the Institute of Physics at TUB, Boersch was a leader in electron microscopy, with side interests in x-rays and laser spectroscopy. Although he may have had links with the Physikalische Technische Bundesanstalt (PTB) in Berlin, Germany’s equivalent of the UK’s National Physical Laboratory, he had never worked in solid state physics, let alone metrology. Boersch must have been impressed by Sauerbrey’s originality and serious preparation.
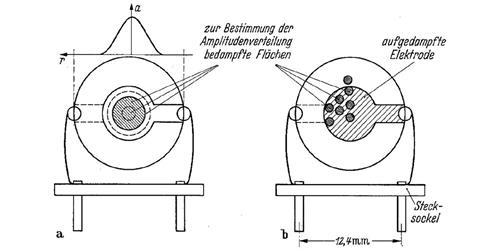
Sauerbrey settled on the study of the vibrations of disc-shaped AT-cut quartz crystals obtained by slicing at 35.17° to the crystallographic axis. With metal electrodes deposited on the upper and lower faces, and mounted on springs, the crystal could be made to vibrate in thickness shear mode, perpendicular to the axis of the disc. An optical system produced interference fringes on the disc’s surface, allowing the precise motions to be observed. Sauerbrey designed the electronic circuits needed to drive and control the oscillations, and he set up the vacuum systems in which to do his measurements. While in his thesis he thanks a single ‘master technician’ for support, he clearly had outstanding mechanical and electronic skills.
He also derived a mathematical model to predict the behaviour of the crystals. He confirmed it by vacuum depositing metal films onto two identical crystals using masks to set the relative ratio of deposition and its location. Finally, he validated his results using a delicate torsion vacuum ultramicrobalance, read with a mirror galvanometer. This confirmed that his vibrating crystals could weigh 0.1 ng of material.
Sauerbrey reported his initial findings at a meeting of the German Physical Society in Heidelberg in 1957; the abstract includes his now eponymous equation. A full paper appeared in 1959, his thesis in 1963. Now a research associate, he also worked on more conventional topics. Having married fellow physics student Helga Wenzel, perhaps he feared the precarity of the road to a permanent position. He joined the PTB, first as a scientist and later as a highly effective administrator and leader.
His QCM, however, immediately caught the attention of surface scientists who began to use it to measure adsorption. With no patent or licensing to think about, commercial devices were available by 1965. As QCMs spread, used from vacuum to biological media, some asked: What exactly did these devices measure? It wasn’t just mass, but a much more informative convolution of the amount, stiffness and internal dynamics of whatever was in contact with the crystal. QCMs continue to find new applications.
To his children, Sauerbrey would speak quietly of having had only a single good idea in his life. But like all great mashups, it inspired a whole lot more.
Acknowledgments
Christoph Salzmann for inspiration and brilliant use of the Berlin phone book; Erik Strub and Sonja Bazhenova for locating and scanning Sauerbrey’s thesis; Pastor Ute Sauerbrey for sharing her memories of her father and his portrait.
References
G Sauerbrey, Z. Phys., 1959, 122, 206 (DOI: 10.1007/BF01337937)
G H Sauerbrey, Experimentelle Untersuchung der Dickenscherungs-Schwingung piezoelektrisch angeregter Quarzplatten. Thesis, TUB, 1963









No comments yet