Turbo-charged Diels-Alder reaction
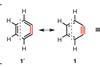
A new method for generating arynes from alkynes has been discovered via a Diels-Alder reaction

A new method for generating arynes from alkynes has been discovered via a Diels-Alder reaction