Fifty years ago, Sir Hans Krebs was awarded a Nobel prize for his contributions to biochemistry. Elizabeth Willcocks reflects on his life.
Fifty years ago, Sir Hans Krebs was awarded a Nobel prize for his contributions to biochemistry. Elizabeth Willcocks reflects on his life.
Sir Hans Krebs always considered himself to be a lucky man. His luck was both personal - he was a Jew who escaped Nazi persecution - and professional. But luck aside, Krebs was also, right up until his death in 1981, a hard-working and dedicated scientist and one of the world’s most eminent biochemists.
Krebs was born in Germany in 1900, the son of George Krebs, an ear, nose and throat surgeon, and his wife Alma. A shy and solitary young boy, with a keen thirst for knowledge, Krebs was anxious to conform to his parents’ expectations. He was particularly keen to please his father who, he felt, doubted his intellectual potential; by the age of 15 Hans had decided to follow him into the medical profession.
Krebs’s schooldays ended six months prematurely because of his conscription into the German army in September 1918, although he was allowed to sit an ’emergency’ higher school examination before he left. And here, perhaps, came his first stroke of luck - two months later World War I ended, leaving him free to return to his study and start university. Had Krebs been only a few years older, the history of biochemistry may have been very different.
Early inspiration
In December 1918, Krebs began his medical studies at the University of G?ttingen, and after a year he transferred to Freiburg University. It was here that his lecturers, in talking about their own work, first inspired him to dabble in scientific research. The first opportunity to try his hand at research came when he took up a summer position in 1920 with Wilhelm von M?llendorf, investigating the physico-chemical principles of tissue staining. The work resulted in his first published paper and, perhaps more importantly, alerted Krebs to the importance of chemistry to the understanding of biology, sowing the seeds for his later interest in biochemistry.
Despite his obvious interest in research, Krebs was all-too aware that fundamental research was not an activity for which people were paid. In the 1920s, research was regarded as a privileged occupation for those who were financially independent. University researchers earned their living by teaching or, if they were medically trained, by looking after patients. Any research was done in their spare time.
So, with research offering limited financial prospects, Krebs returned to his medical studies and passed his final examinations in December 1923. To obtain a licence to practise medicine, Krebs had to work in a recognised hospital for a year. However, the economic conditions in Germany during the early 1920s meant that paid work was hard to come by and he eventually secured an unpaid position at the Third Medical Clinic in the University of Berlin.
Krebs deliberately chose a position in general medicine because he felt that it offered the best opportunity to develop his research interests. During his 12 months at the Third Medical Clinic, he became ever-more convinced of the importance of chemistry to medical research, and also of his own inadequate chemical knowledge. With this in mind, and his 12 months up, Krebs moved on to the department of chemistry at the Pathological Institute of the Charit? Hospital, Berlin, to take some informal training in chemistry and biochemistry.
Krebs’s father continued to support him during this time, despite his concerns about the prospects for earning a decent living from research. However, in 1925 Krebs was offered his first paid research position - in the laboratory of the Nobel laureate Otto Warburg.
A chance meeting
Krebs admitted that his appointment as an assistant to Warburg - whom he called ’the most remarkable person I have ever been closely associated with’ - came by way of a chance meeting. A close friendship between Warburg and Krebs’s friend Bruno Mendel, sparked at a dinner party held by the great Albert Einstein, led Mendel to recommend Krebs as a potential collaborator for Warburg at the Kaiser Wilhelm Institute for Biology in Berlin.
Krebs held a deep respect for Warburg, admiring his dedication to advancing scientific knowledge, the long hours he worked (8am-6pm, six days a week, was the norm at the Institute) and his intellectual honesty. Krebs found the whole environment stimulating, and from Warburg he learned many techniques that would help him in his later work. During his three years there he became acquainted with many other famous names in science - including Fritz Lipmann, with whom he later shared his Nobel prize.
Although the position at the Kaiser Wilhelm Institute was paid, it was a modest income and Krebs lived his life according to his means. But money was not Krebs’s motivation - he and his colleagues were driven by a dedication to their work. At any time Krebs could have branched off into a much more lucrative medical career, but his dedication to research and the pursuit of knowledge was clear.
Krebs’s time with Warburg was fruitful - he published 16 papers in total during his four years at the Kaiser Wilhelm Institute - but it could not last forever. By 1929, Warburg had begun to hint to Krebs that he should move on. After a brief stint at the Municipal Hospital of Altona, where Krebs fitted his research work around heavy clinical duties, he took up a post as an assistant in the department of medicine at the University of Freiburg in 1931.
Although Krebs’s position at Freiburg required him to take on some clinical responsibilities, it was here that he made his first crucial discovery - the ornithine cycle for urea synthesis. Luck was again on his side - the discovery of the ornithine cycle in 1932 established his reputation among the international scientific community just one year before Hitler came to power and the rise of the Nazi regime forced Krebs to seek a post outside of Germany.
Merry England
In April 1933, Krebs received official confirmation of his dismissal from Freiburg under the law for the reconstruction of the professional civil service - in other words, the removal of all non-Aryans and anti-Nazis from professional occupations. With his international reputation recently established, Krebs was welcomed with open arms by Cambridge University’s biochemistry department within a matter of months. And so began his successful career in England.
It was not long before Krebs felt at home in Cambridge and the university’s biochemistry laboratory - which he called a ’hive of activity’. He described the English way of life as suiting him ’down to the ground’ and was touched by both the warm hospitality and generosity of the friends he made in Cambridge and what he felt was a lack of class, race and religious prejudice compared with what he had experienced in his home country.
Krebs had been fortunate in bringing all his equipment with him from Germany. This enabled him to continue the work that he had started in Freiburg, including some experiments concerning the fate of dicarboxylic acids in kidney tissue - work that helped him to conceive of the famous Krebs cycle. In time he was given a position as a demonstrator in the laboratory - the lowest position on the Cambridge academic ladder - and through this he introduced some of Warburg’s own techniques for the first time into undergraduate classes.
In 1935, Krebs was offered a post as a lecturer in pharmacology at the University of Sheffield. Attracted by the offer of more space - the Cambridge laboratory was a bit cramped - and double his salary, he made the move north. It was in Sheffield that Krebs met Margaret Fieldhouse, whom he married in 1938, and where he would eventually spend what he described as ’19 happy years’.
The cycle
During his time at Sheffield, Krebs performed some of his most important experiments and it was here that he finally conceived of the ’Krebs cycle’, which describes some of the chemical reactions that occur when food is burned in the body to produce energy.
By the time that Krebs started to think about the combustion of food, many other scientists had already discovered chemical reactions that appeared to be involved, but the full significance of these discoveries had not yet been realised.
Along with many of his contemporaries, Krebs knew that the overall process involved one molecule of glucose reacting with six molecules of oxygen to produce six molecules of CO2 and six molecules of H2O. They were also aware that the principles of chemistry did not allow all six molecules to react at once and that this pointed to a multi-step combustion process. Scientists also knew that glucose could not be burned until it had been fermented and that it was pyruvic acid - the final product of fermentation - that was actually burned.
Albert Szent-Gy?rgyi, a Hungarian-American biochemist, had discovered that succinic, fumaric, malic and oxaloacetic acids all burn readily and that succinic acid can be converted to oxaloacetic acid via the other two acids (Scheme 1). Szent-Gy?rgyi concluded that these dicarboxylic acids must be intermediates in the combustion process. However, the fact that the acids bore little resemblance to food molecules and that there appeared to be no obvious pathway for converting oxaloacetic acid to the end products of combustion - CO2 and H2O - masked the significance of Szent-Gy?rgyi’s work.
The intermediate steps of the Krebs cycle
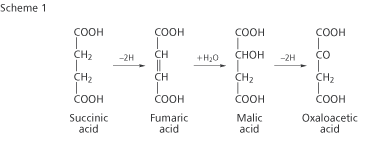
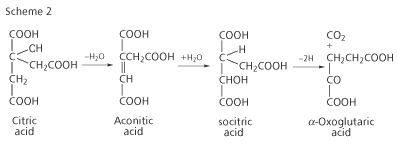
The second vital clue for Krebs came from the work of two German biochemists, Franz Knoop and Carl Martius, who discovered that citric acid could be converted by a series of reactions to ?-oxoglutaric acid (Scheme 2). Scientists knew that ?-oxoglutaric acid could be oxidised to succinic acid, and so, by linking Schemes 1 and 2, the route by which citric acid could be converted to oxaloacetic acid was discovered. But it was still not clear what these reactions had to do with the combustion of food.
Krebs was convinced that the reactions discovered by Knoop, Martius and Szent-Gy?rgyi played a vital part in the combustion of food, because they were the only ones of the many dozens of substances investigated that burned at the same rate as food. He also knew, from earlier work by two scientists, J. Thunberg and J. H. Quastel, that malonic acid - a substance that specifically inhibits the conversion of succinic acid to fumaric acid, one of the steps that Szent-Gy?rgyi had discovered, inhibited combustion in living cells. To Krebs, this strongly suggested that the sequence of reactions converting citric acid to oxaloacetic acid played an important part in the oxidation of food.
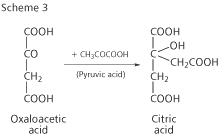
Krebs suspected that oxaloacetic acid might react with pyruvic acid to form citric acid, thus forming a complete cycle for the combustion of foodstuffs. He admitted that, by another stroke of luck, his discovery of the ornithine cycle alerted him to the possibility of a cyclical process, and his hunch was right - he proved that citric acid could be synthesised from pyruvic and oxaloacetic acids (Scheme 3) at a rate that was compatible with combustion in living cells. The Krebs cycle was born.
In 1937 Krebs felt that he had amassed enough evidence to support his cycle, and he sent his work in a letter to the editor of Nature. Famously, the paper describing the work that was later to win Krebs the 1953 Nobel prize for physiology or medicine, was rejected five days later. The paper was eventually published in the Dutch journal Enzymologia, but Nature wasted no time in sending Krebs its congratulations when he received his Nobel prize.
Krebs’s Empire
While Krebs was at Sheffield, the university opened a biochemistry department, making Krebs its head. In 1945 he became Sheffield’s first professor of biochemistry and in the 1950s the University established an undergraduate degree course in the subject. In 1944, the Medical Research Council (MRC) also established a research unit for cell metabolism at Sheffield, which Krebs was to direct. With his MRC funding, Krebs rarely wanted for money or space - the university even converted the top floor of the old Scala cinema into a laboratory for him and his coworkers, which the locals jokingly called Krebs’s ’Empire’. It was also through the MRC unit that Krebs gathered his loyal team of colleagues, some of whom continued to work with him after his retirement.
In 1954, Krebs moved from Sheffield to become the Whitley professor of biochemistry at Oxford University, taking his MRC unit with him. There, Krebs became involved with the university’s administrative issues, including the concern that the college science fellows received more money than their colleagues with no college fellowships. Joel Mandelstam, who was a colleague of Krebs at Oxford, remembers that he ’always had good ideas about how the university and department should be run’, but that he became frustrated by an institution that was ’very set in its ways’.
In 1967, Krebs retired from his post at Oxford, but he was keen not to slip into ’professional inactivity’, and he promptly moved to a position at the Nuffield Department of Clinical Medicine, at the Radcliffe Infirmary in Oxford, again taking his MRC unit with him. When asked why he carried on working into his ’retirement’, and why he did not use the time to enjoy himself, Krebs answered that someone asking such a question could not have experienced the profound enjoyment and intellectual satisfaction that his working life had provided him with. Science and research remained, as it always had been, his life’s love.
Krebs admitted to having been a hard task-master and poor at giving praise where it was due, but he inspired many people, including some scientists who started with him as technicians and worked their way up to become professors at other universities. Luck may have played a part in this story - of one of the world’s most important biochemists - but Krebs’s hard work and dedication to advancing scientific knowledge is perhaps the real reason why his legacy is so immense.
Source: Chemistry in Britain
Further Reading
- H. Krebs, Reminiscences and reflections. Oxford: OUP, 1981.
- D. H. Williamson, Biochem. Soc. Trans., 1999, 9, 1.
- H. Gest, Biochemistry and molecular biology education, 2002, 30, 9.
1. Fritz Albert Lipmann 1899-1986
Sir Hans Krebs shared the 1953 prize for physiology or medicine with the German-born biochemist Fritz Lipmann. Lipmann’s contribution to unravelling the mystery of how food burns in the body was his discovery of coenzyme A (CoA) - a crucial link between glycolysis, the first stage in the process, and the Krebs cycle.
When Krebs put together the citric acid cycle in 1937, there remained one question: how did pyruvic acid - the final product of glycolysis - enter the cycle and react with oxaloacetic acid to form citric acid? It was more than 10 years before Lipmann found the answer - a small molecule called coenzyme A was necessary for the reaction between oxaloacetic acid and pyruvic acid, forming citric acid, to take place. Two years after Lipmann’s discovery, another German biochemist, Feodor Lynen, demonstrated that pyruvic acid reacts with CoA to form acetyl CoA. It is the acetyl CoA that reacts with oxaloacetic acid to form citric acid and CoA. Lynen’s contributions to biochemistry also won him a Nobel prize in physiology or medicine, in 1964.
Lipmann’s interest in chemistry and biochemistry was ignited by what he called a ’dramatic chemistry course’ that he had taken during his preclinical year of medical study. In 1926, he took up a post as an assistant to Otto Meyerhof at the Kaiser Wilhelm Institute, Berlin, where, in 1927, he met Krebs. At the time, Krebs was working as an assistant to Otto Warburg at the Institute, and he remembers ’pleasant and stimulating times’ spent there with Lipmann.
In 1930, Lipmann left Germany to work at the Rockefeller Institute in New York and, after a brief spell in Copenhagen, emigrated to the US in 1939. After Krebs left Germany, he met with Lipmann only once more before they were reunited at the 1953 Nobel prize ceremony.
During his lifetime, Lipmann made important contributions to many other areas of biochemistry, including the metabolism of fibroblasts, the role of glycolysis in embryo cell metabolism, the chemical nature of unusual phosphate derivatives and the biological mechanisms of peptide and protein synthesis.
2. The Krebs cycle
The original cycle of reactions proposed by Krebs in 1937 - known as the Krebs cycle, the citric acid cycle and the tricarboxylic acid cycle - represents the second of three steps that convert glucose and other fuel molecules in the body into energy in the form of adenosine triphosphate (ATP). The first step is a series of reactions called glycolysis - literally, sugar-splitting - which break down glucose to form pyruvic acid ready to enter the Krebs cycle.
Pyruvic acid is activated by combining it with coenzyme A (CoA) to form acetyl coenzyme A (acetyl CoA). Acetyl CoA then combines with oxaloacetic acid to form citric acid (hence citric acid cycle) and CoA. Citric acid is converted back to oxaloacetic acid through a series of other tricarboxylic acids - hence the cycle’s third name.
One complete turn of the cycle produces two molecules of CO2 and eight hydrogen atoms, and also the biological intermediates NADH (reduced nicotinamide adenine dinucleotide) and FADH2 (reduced flavine adenine dinucleotide) which are used in biosynthesis. Only a few ATP molecules are produced by glycolysis and the Krebs cycle - the majority are produced by the third step in the process, known as oxidative phosphorylation.







No comments yet