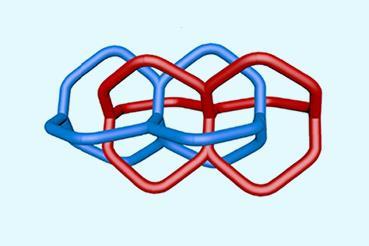
![Cyclo[48]carbon [4]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xAny/4/6/3/542463_indexady6054_articlecontent_v2_18june3_70174.jpg)
Chemists have produced a new ring molecule of pure carbon that is stable enough to be studied in solution – as long as it is hooked up with other rings.
We know pure carbon as diamond, graphite, graphene and fullerenes. Now, the University of Oxford team of Harry Anderson with Yueze Gao, in collaboration with three other UK institutes, has produced the first pure carbon ring that is stable enough to be bottled and investigated using conventional spectroscopy methods.
All previous carbon rings were produced by atom manipulation on solid substrates requiring extremely low temperatures in a vacuum. In 2019, researchers at the University of Oxford and IBM Zurich pioneered this methodology and reported the ring-shaped molecule C18, which was quickly followed by similar rings with six to 26 carbon atoms.
Like the earlier rings, the new molecule, C48, is made of alternating single and triple bonds between the carbon atoms, forming a polyyne. As these bonds would quickly react to form cross-links between molecules, the researchers had to keep the molecules apart. They achieved this by running three bulky macrocycles around the carbon loop, creating a catenane.
With this arrangement, the carbon ring turned out to be reasonably stable in a dilute solution at 20°C, with a half-life of 92 hours. This enabled the team to study the molecular ring with NMR and Raman spectroscopies, as well as mass spectrometry, which confirmed the identity of the C48 ring and its structure as a perfectly symmetrical molecular circle, with all 48 carbon atoms exactly equivalent.
![Cyclo[48]carbon [4]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xany/4/6/4/542464_ady6054_articlecontent_v2_18june3_795315.jpg)
Part of the reason for its relative stability is also the size of the ring, which means that the polyyne chain, which thermodynamically favours a linear structure, doesn’t have to bend too much. The bond angle in a polygon of 48 sides is 172.5°, which is within the range of the wriggle room that a normal open-chain polyyne would move in.
Thanks to its relatively comfortable conformation, the cyclocarbon ring can even survive without the protective catenane entanglement for long enough to have its identity confirmed, and to refute any objections that the catenated version isn’t a pure carbon molecule.
This was more than the researchers had hoped for. ‘The main surprise for me is that “naked” cyclo-C48 is stable enough to survive for minutes in solution at room temperature,’ Anderson says. ‘The next step will be the synthesis of receptors that can completely encapsulate C48, so that we can characterise it in the solid state.’
Przemyslaw Gawel of the University of Warsaw, Poland, who was involved in early attempts to produce cyclocarbons in Anderson’s lab 10 years ago, welcomed the breakthrough. ‘The fact that we can now study these elusive carbon rings in solution is a major leap forward, one that brings us closer to understanding the unique world of linear carbon allotropes and the fundamental versatility of carbon as an element.’
References
Y Gao et al, Science, 2025, DOI: 10.1126/science.ady6054













No comments yet