
Burning a candle doped with mixed metal precursors can produce carbon soot nanoparticles containing up to 25 metals. The researchers behind the work said that the approach could be a promising method for electrocatalyst development.
Making high-entropy metal-containing nanomaterials generally requires extreme conditions, including high pressure and temperatures above 1000K, as well as highly specialised equipment. In this study, a team of researchers based in China and Australia demonstrated a flame synthesis process whereby paraffin wax was doped with organometallic precursors before the candle was lit. Once the candle was ignited, the metal precursors migrated with the paraffin wax along the wick, by way of capillary action, from the core to the outer flame zone.
The flame naturally produced a stable and wide temperature gradient from the centre of the flame, around 1800K, to the stainless-steel soot collector placed around 20mm away from the burning wick. The intense heat of the flame enabled homogeneous bonding between dissimilar metallic elements, generating tiny metal-containing carbon soot nanoparticles.
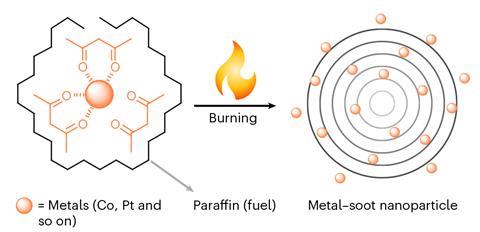
The researchers studied the physicochemical properties of the nanoparticles using electron microscopy and found them to be around 40nm in diameter, with an onion-like layered structure, large surface area and low electrical resistance.
Using this approach, up to 25 metals were successfully incorporated into a single soot nanoparticle, and the researchers said the soot nanoparticles’ composition could be fine-tuned and the method easily scaled up, making it a promising approach for many catalytic reactions.
The team also demonstrated the potential application of a high-entropy metal nanoparticle containing iron, cobalt, nickel, copper and palladium in the electrocatalytic production of hydrogen peroxide.
References
Z Liu et al, Nat. Chem., 2025, DOI: 10.1038/s41557-025-01894-w















No comments yet