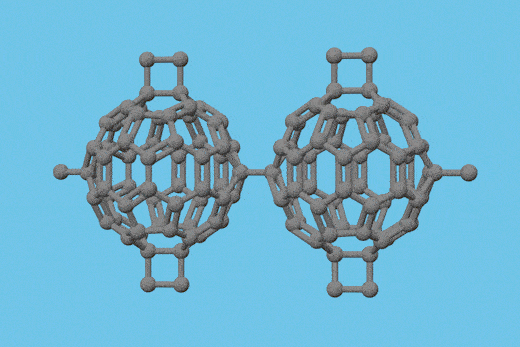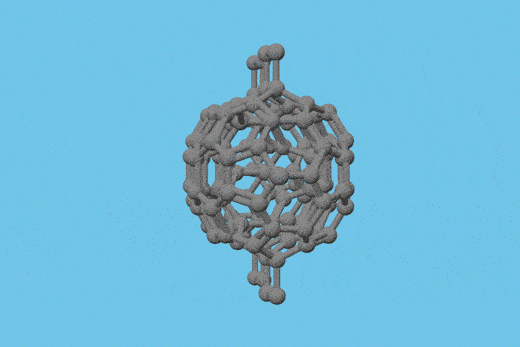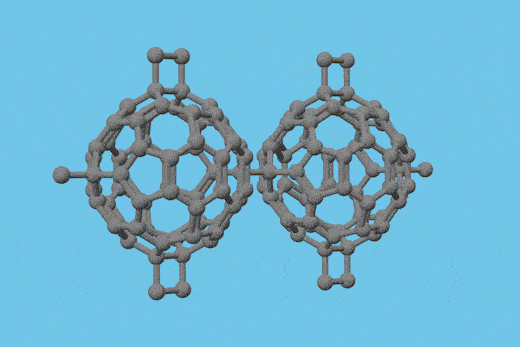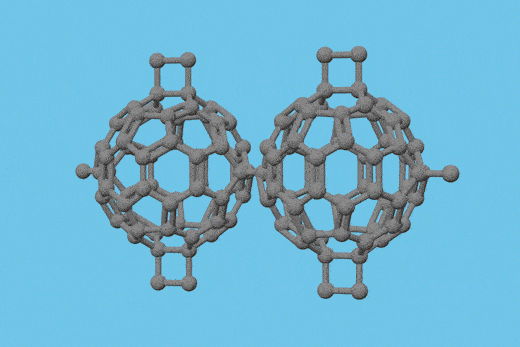
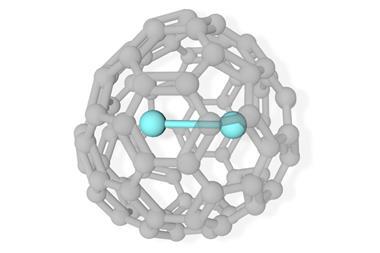
Scientists in China have isolated a double heptagon-containing C70H6 fullerene from the soot of a benzene and acetylene mixture that has combusted in low-pressure conditions.1
Surprisingly, the fullerene’s carbon core (C70) is identical to the carbon core of a C70Cl6 fullerene the Xiamen University team had synthesised with an arc discharge technique.2 This is the first time that the same nonclassical fullerene cage has been detected in the products of the two different processes. Before now, none of the heptagon-containing fullerenes synthesised by such methods have been similar, leading researchers to debate the different fullerene formation mechanisms involved.
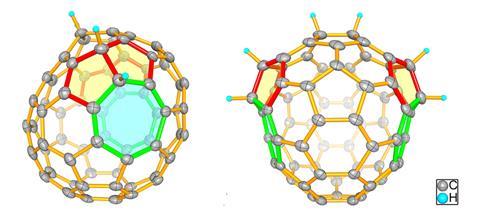
The team confirmed that the C70 cores from both processes are identical using single crystal x-ray diffraction. With the support of density functional theory calculations, they also demonstrated that the C70H6 fullerene is more stable than the C70Cl6 fullerene.
Thus, the scientists infer that fullerene formation in both the arc-discharge and combustion processes must share similar features. They also propose that the more stable C70H6 fullerene be used to study the properties of the C70 cage structure and double heptagon formation.
Since derivatives of fullerenes are used in polymer and perovskite solar cells, insights into the formation mechanisms and properties of fullerenes could help improve the performance of these devices.
References
1 F-F Xie et al, Chem. Commun., 2022, 58, 9814 (DOI: 10.1039/d2cc03707f)
2 Y-Y Zhong et al, Angew. Chem., Int. Ed., 2019, 58, 14095 (DOI: 10.1002/anie.201902154)









No comments yet