
Insects could provide an alternative way of synthesising oxygen-doped molecular nanocarbons that are notoriously difficult to prepare in the lab. Dubbed ‘in-insect synthesis’, the researchers, based in Japan, said the technique could generate new opportunities for the discovery, development and application of non-natural molecules, such as nanocarbons.
Kenichiro Itami, a synthetic chemist at Riken Center for Sustainable Resource Science in Japan, who led the study, admits that in-insect synthesis will sound ‘pretty crazy’ to most organic and synthetic chemists. ‘Not many people are doing insect chemical biology or seeing the relationship within a synthetic molecule or functional molecule with an insect,’ he adds.
This is an alternative, crazy option for synthetic chemists, for making molecules
Kenichiro Itami, Riken
The researchers first looked at using silkworms in their work but found that the substrate they were using, a belt-shaped molecular nanocarbon known as methylene-bridged [6]cycloparaphenylene ([6]MCPP), was inherently toxic to them. Then the first author of the paper, Atsushi Usami, came to Itami’s office with an idea. ‘[He said] there’s one interesting insect – the tobacco cutworm. He mentioned that it is a serious pest, they eat a lot and their life cycle is pretty fast (35–40 days),’ says Itami. ‘We thought that maybe [it] would be strong enough for our purpose.’
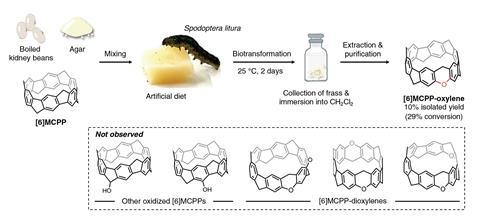
To carry out the in-insect synthesis they designed a diet for the tobacco cutworm larvae of boiled kidney beans and agar with nanocarbon substrate. ‘We let them eat, waited for two days, and then they produced lots of frass. We collected the [frass], and then [carried out extraction] using an organic solvent, [to] get a solution of the crude product. Then we took thin layer chromatography and saw this one little spot that was different from the starting material. It was not super high yielding in the beginning, but once we isolated it from the mass spec we knew there was an oxidant insertion product.’ The researchers named the oxygen adduct [6]MCPP-oxylene.
To verify whether the oxygen-doping was derived from intestinal bacteria, enzymes in the cutworm or both, they carried out biotransformation using intestinal bacteria as the target but ultimately found that bacteria were not involved. Instead, they found that two cytochrome P450 enzymes, CYPX2 and CYPX3, were responsible for the transformation. Computer simulations showed that these enzymes could simultaneously, and stably, bind two [6]MCPP molecules and directly insert an oxygen atom into a carbon–carbon bond to produce [6]MCPP-oxylene.
‘These particular molecules are almost impossible to synthesise by influx synthesis … it’s a lengthy, low-yielding process,’ says Itami. ‘If we can transform the already created nanocarbon in a single shot with something like insect synthesis that will be extremely useful, because you can derivatise lots of different kinds of molecule for discovering functional molecules or even biological active molecules – that is the uniqueness of our reaction.’
‘This is an alternative, crazy option for synthetic chemists, for making molecules,’ Itami says. ‘I won’t say that in-insect synthesis will completely replace in-flask, but it is an option for derivatising the molecule that we synthetic chemists synthesise over years, worldwide.’
Scale-up tricky
Itami adds that because the process does not kill the cutworms they can be reused, although their synthetic abilities declined over subsequent rounds. The main challenge with the approach, he says, is scalability. ‘If you want to make 1kg of the molecule, I cannot imagine what kind of processes or machines or equipment we would need.’
Milo Shaffer, a materials chemist at Imperial College London, says the work was a ‘fun experiment’ but admits that he wasn’t sure if the researchers were suggesting this approach could be a ‘viable synthetic pathway for the future’. ‘I guess they were excited by the selectivity of the oxidation, because you might have thought it would stick oxygen groups in various places because, from a biological point of view, I wouldn’t have thought the organism has a strong preference for where the oxygen goes so you might say it’s a bit surprising it’s so selective.’
‘Then they tried on the different ring sizes, and it only seems to work for one ring size, which is quite surprising, because you might think shouldn’t it work for all of them, or at least some of them?’ Shaffer says that what the paper was missing was a comparison with other oxidation routes. ‘If you were trying to do that just purely synthetically, without going to the trouble of growing your caterpillars and making them eat it and then excrete it and then separate it out, you could try all sorts of conventional oxidation reagents, and [look at] whether any of those would have produced similar products.’
However, he says that if no one has proposed this synthetic route to make a product, then ‘conceptually maybe that’s an interesting point to make’. ‘In the end I don’t know why you want the whole organism … Because you could just take the enzyme; there’s lots of people doing amazing things with extracted enzymes, manipulated enzymes, redesigned enzymes that work on non-biological substrates of various kinds.’
References
A Usami et al, Science, 2025, 388, 1055 (DOI:10.1126/science.adp9384)














No comments yet