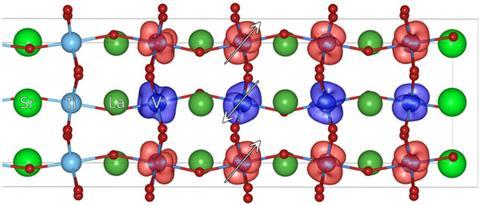
Stacks of insulating transition metal oxides could effectively convert sunlight into electricity. A team centred at the Technical University of Vienna in Austria, has calculated that layering LaVO3 on a SrTiO3 base could also deliver advantages conventional semiconductors can’t.1 ‘Using LaVO3/SrTiO3 for photovoltaics is a totally new idea,’ says Giorgio Sangiovanni, who led the team and has now moved to the University of Würzburg, Germany.
In 2002 scientists found LaAlO3/SrTiO3 interfaces can produce conductive ‘two-dimensional electron gases’ commonly found in semiconductors, despite the two oxides normally being insulators.2 Sangiovanni’s team realised this could benefit solar cells, where photons excite an electron from a semiconductor’s valence band to its conduction band. The resulting electron, and the positive hole left behind, move in opposite directions towards the cell’s anode and cathode, creating electrical current. However, photovoltaic materials only absorb photons with more energy than the gap between bands, which can limit cells’ efficiency. At over 3eV, LaAlO3/SrTiO3’s bandgap is too large Sangiovanni says, but the 1.1eV bandgap of the related material LaVO3/SrTiO3 is ‘ideal for solar-cell applications’.
An additional benefit of layered transition metal oxides, Sangiovanni explains, is that they could also separate electrons and holes. ‘Many of these systems are polar, with nominally positively and negatively charged layers,’ he says, creating an electric field that pushes the electrons and holes apart. And with thin enough LaVO3 the interface also behaves like a metal, which could extract solar cells’ generated electricity without needing extra electrodes.
Meanwhile, the highest efficiency photovoltaic cells capture more solar energy by stacking semiconductor materials with progressively narrower bandgaps such as GaAsP2/GaAs/Ge. Sangiovanni’s team has calculated that transition metal oxides could do that too, for example using LaFeO3/LaVO3/SrTiO3 layers.
Jean-Marc Triscone at the University of Geneva, Switzerland, who also studies oxide interfaces, calls this study a ‘nice idea’. But he’s concerned that the electric field may be very low in the thick layers needed for solar cells. He also worries that there could be too many unwanted electron–hole recombinations in oxide materials for photovoltaic use, partly due to crystal defects. ‘Will we be able to achieve the required quality?’ he asks. ‘We should certainly try.’
Though Sangiovanni concedes that this technology is at a very early stage, his Würzburg colleagues are attempting the first physical measurements on it. ‘Now it is time for experiments,’ he says. ‘We hope the results will be as positive as we have predicted.’






No comments yet