Japanese researchers show that lead can be incorporated into an aromatic ring, the heaviest metal so far to do this
Scientists in Japan have successfully incorporated an atom of lead into an aromatic molecule - the heaviest metal so far to be ’aromatised’. The finding could result in novel structures with useful electron-donating characteristics, with potential applications in areas such as catalysis or optoelectronics.
Forming a stable aromatic ring with an element such as lead is difficult because of the wide discrepancy in the size of the electronic orbitals of the metal and the carbon atoms (6p and 2p orbitals, respectively) in the ring. A certain degree of overlap is needed for electrons to be shared evenly around the ring - a requirement for aromaticity. If one orbital is huge and the other tiny, getting overlap is a tall order.
Masaichi Saito, of Saitama University, and colleagues took as their starting material hexaphenylplumbole, a five-membered non-aromatic ring compound containing four carbons and one lead, with a phenyl group attached to each carbon and two phenyls attached to the lead. They then reduced the compound with lithium, which had the effect of substituting the two phenols bound to the lead with two lithium ions.
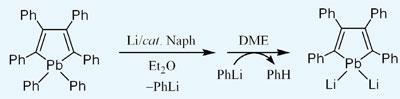
The Li-Pb bond is much more polar than the Pb-C bond, and the lead becomes anionic, effectively receiving two electrons from the lithium. This pair of electrons is available to delocalise in the ring, joining the four other electrons from the carbon skeleton to make the molecule aromatic.
Having demonstrated that it is possible to make 2p and 6p orbitals overlap sufficiently to create an aromatic molecule, Saito says that this could give rise to new materials with potential applications in fields such as catalysis. ’In particular the dianionic character of this molecule could make it useful as an electron donor,’ he says.
David Scheschkewitz, an inorganic chemist at Imperial College London, describes the new research as ’a very nice piece of work’. Scheschkewitz says that the isolation of the first stable aromatic compound with a lead atom as part of a delocalised cyclic pi-electron system is a remarkable synthetic achievement. ’The study demonstrates impressively how the carbene-like character of the heavier atom in group 14 metalole dianions increases when going down the group. The compound may thus have an interesting coordination chemistry towards transition metals. In view of the importance of metalole-related repeat units in optoelectronic materials, a future application of the reported compound is clearly on the cards,’ he adds.
Simon Hadlington
References
10.1126/science.1183648)






No comments yet