A cheap and non-toxic way to selectively separate lithium isotopes – without using huge amounts of mercury – could help supply the future fuel needs of fusion reactors. The new approach uses a porous vanadium oxide compound that acts as an isotope-sensitive filter, sequestering the lighter lithium-6 isotope, while allowing lithium-7 to pass through faster. ‘If nuclear fusion plans become a reality, we’ll need to produce tonnes of lithium-6 per day and I think that this would be a very competitive method for doing so,’ says Sarbajit Banerjee of ETH Zürich in Switzerland and Texas A&M University in the US who led the study.
Promising advances in nuclear fusion have sparked renewed interest in this technology as a sustainable energy source and lithium-6 plays a key role in the process. But the standard method for isolating this isotope from the much more abundant lithium-7 isotope requires a lot of mercury to take advantage of the fact that lithium isotopes have varying solubility in amalgams. The demand for so much of this heavy metal led to the conventional approach being banned in the US over 60 years ago.
‘The most common fusion process involves a deuterium–tritium reaction, but tritium is pretty rare, so the idea is to generate it on site,’ says Banerjee. He explains that this is done by bombarding a lithium-containing material with neutrons. ‘The light stable isotope lithium-6 is a superb neutron absorber and is therefore useful for the production of tritium,’ comments Abby Kavner from the University of California, Los Angeles, who wasn’t involved in the study.
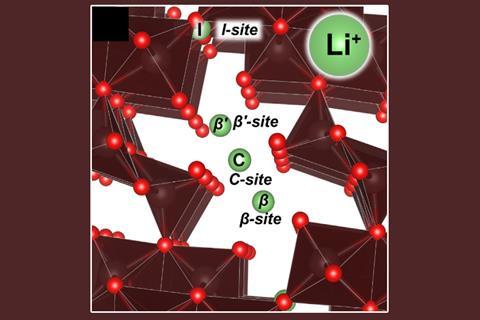
Banerjee points out that only about 7% of all the lithium in the world is lithium-6. That’s why his team have focused on extracting this important isotope from fracking and oil drilling wastewater. ‘We’re flowing water streams through something that looks like a desalination cell and are electrochemically capturing the lithium inside empty spaces of the vanadium oxide,’ Banerjee says. He explains that the porous material acts as a cathode that captures the positively charged lithium ions from the solution within its tunnelled structure when a voltage is applied to the system. The process can separate the two isotopes because the lithium-6 ions bind more strongly to the vanadium oxide, while the lithium-7 ions are able to pass through. ‘If you think of the bonds between V2O5 and lithium as a spring, you can imagine that lithium-7 is heavier and more likely to break that bond, whereas lithium-6, because it’s lighter, reverberates less and makes a tighter bond,’ explains co-first author Andrew Ezazi of Texas A&M. The researchers are now taking steps to scale up their process.
Kavner adds that vanadium plays a key role in this process due to its electrochemical properties. ‘The result is an inexpensive, non-toxic electrochemical filter that can not only isolate lithium ions from multicomponent solutions, but also control the relative rates of migration of the lithium isotopes through the filters,’ she says. ‘The resulting single-pass isotope enrichment factors improve by almost 15%, which seems modest but compounds during the multi-pass enrichment process.’
‘You have to run the process multiple times – probably 20 times or more – looping it around to get increasingly pure lithium-6,’ explains Banerjee. ‘And of course it depends on the lithium source you’re using. If you use a dirty water source, that’s a bit more complicated. If you use a pretty clean source of highly enriched lithium brine and all you want to do is isotope separation, that’s easier.’ Banerjee notes that under ideal conditions, it’s possible to achieve 30% lithium-6 enrichment within 25 cycles and that an enrichment of up to 90% can be reached after 45 cycles.
References
JL Carrillo et al, Chem, 2025, DOI: 10.1016/j.chempr.2025.102486










No comments yet