High-resolution electron microscopy films individual metal atoms as they move around and react inside a carbon nanotube
In a remarkable home movie, an international team of researchers has filmed individual metal atoms as they move around and react within the confines of a carbon nanotube. As well as demonstrating the power of the imaging technique, the work has shown that the interior of carbon nanotubes may not be as inert as previously assumed.
Andrey Chuvilin from the University of Ulm in Germany and colleagues trapped single atoms of the heavy metal dysprosium within hollow fullerene spheres made up of 82 carbon atoms, and enclosed a series of these dysprosium-seeded cages within single-walled carbon nanotubes, with the fullerenes stringing themselves along the nanotube like peas in a pod.
Using a technique called aberration-corrected TEM (transmission electron microscopy), the team was able directly to observe the dysprosium atoms interacting with the carbon atoms of the fullerene and nanotube.
’This technique allows us to use a much lower energy electron beam than in conventional TEM,’ says team member Andrei Khlobystov of the University of Nottingham in the UK. ’With the usual higher energies, the electrons themselves can damage the structures, but here the beam energy is below the threshold for causing damage.’ Any structural changes could not therefore be attributed to the effects of the microscope itself.
The researchers watched as the dysprosium atoms began to chew away at the wall of the fullerene cage, eventually escaping. Neighbouring ruptured cages then fused together to create small nanotubes. Meanwhile, the liberated dysprosium atoms gradually clustered together and then attacked the wall of the main nanotube, causing the wall to break open and then form a new cap at that point.
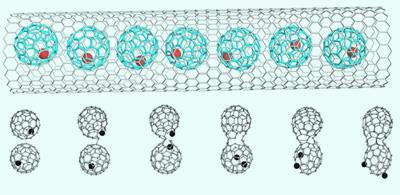
’The breaking of the carbon bonds of the fullerene and nanotube are catalysed by the dysprosium,’ says Khlobystov. ’One dysprosium atom is capable of breaking open the wall of the fullerene, but because the nanotube is thermodynamically much more stable, it takes a cluster of seven or eight dysprosium atoms to catalyse its rupture.’
Khlobystov says that the work demonstrates that aberration-corrected TEM is able to follow complex interactions within carbon nanotubes at an unprecedented level of atomic resolution, where even the individual carbon atoms of the nanotube wall are distinguishable. In addition, the discovery that the wall can be disrupted by species contained within the nanotube was surprising. ’Normally we consider the interior of a carbon nanotube to be a chemically inert - clearly this is not always the case.’
Peter Harris, who uses TEM to study carbon nanotubes at the University of Reading in the UK, is impressed with the work. He says that it ’exploits beautifully’ the use of carbon nanotubes as ’nano-test-tubes’ in showing how dysprosium atoms can catalyse the modification of carbon nanostructures by, for example, promoting the coalescence of fullerene molecules to form short nanotubes. ’The dysprosium atoms can also eat through the nanotube walls, effectively dividing a tube in two,’ adds Harris. ’It’s a very nice piece of nano-engineering.’
Simon Hadlington
References
et alAngew. Chem. Int. Ed., 2009, DOI: 10.1002/anie.200902243






No comments yet