An international team of researchers has developed a new class of anti-flu drug that could prevent new virus strains developing resistance and help control future pandemics while more effective vaccines are prepared. Each year, flu viruses cause up to five million cases of severe illness worldwide, resulting in up to 500 000 deaths.
The preferred drug treatments for flu – neuraminidase inhibitors including Tamiflu (oseltamivir) and Relenza (zanamivir) – treat infection by stopping the viral surface enzyme neuraminidase from interacting with its natural substrate, sialic acid. It is this interaction that releases the virus from an infected cell and allows it to spread to other cells.
The problem, however, is that influenza viruses are constantly evolving and therefore strains have emerged that are resistant to these drugs. There is therefore a pressing need for new drugs that work in different ways.
Now, researchers in Canada, Australia and the UK have developed a new class of mechanism-based covalent compounds that inhibit neuraminidase in such a way that they think it could reduce the chances of flu viruses developing resistance.
'Our plan was to design a molecule that was as close as possible in structure to the natural substrate (sialic acid), because viruses will develop resistance most readily against drugs that radically differ in structure from the natural substrate,' says Stephen Withers, who led the study at the University of British Columbia, Vancouver.
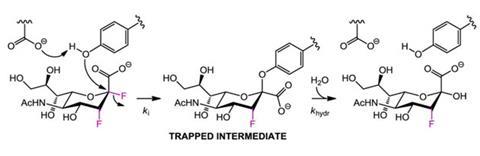
The upshot is the virus cannot mutate its key catalytic residues to stop this covalent attachment. 'If it did so, it would no longer be able to carry out its normal function. Consequently we believe that development of resistance will be much slower, if it occurs at all,' explains Withers.
The compounds have been tested on infected mice, which were completely protected against lethal infection. 'We have already shown that they work well against viral strains that are resistant to Tamiflu or Relenza. We are now embarking on tests to probe whether resistant mutations ever develop against our compounds,' says Withers.
William DeGrado, a pharmaceutical chemist at the University of California, San Francisco, US agrees that this mechanism-based covalent strategy could offer a reduced risk of developing resistance as compared to other existing neuraminidase inhibitors. 'It will be interesting to see whether robust oral bioavailibility can be obtained within this new class of mechanism-based inhibitors,' he comments.
'Oral bioavailability is something we are working on through a pro-drug approach,' says Withers. 'I am very optimistic we can do it.'
References
J-H Kim et al, Science, 2013, DOI: 10.1126/science.1232552






No comments yet