Nobelium is now the heaviest element to have been directly detected as part of a larger molecule. The new nobelium complexes were created as part of a series of investigations charting the chemistry on the edge of the actinide series.
Nobelium is element 102, and is too unstable to exist naturally on Earth. It was first made in particle accelerators in the 1950s, with Swedish, US and Russian teams all claiming to have been the first to have created it. As it has to be produced through nuclear reactions a single atom at a time, nobelium has remained one of the most mysterious elements on the periodic table, with no known uses and very few of its chemical properties confirmed experimentally.
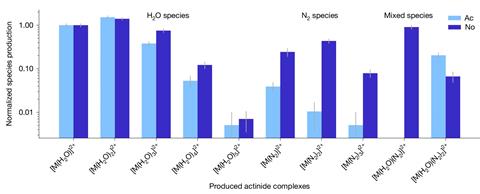
In the new work, a team at Lawrence Berkeley National Laboratory in California, US, first created nobelium by firing a calcium beam into a lead target using a cyclotron particle accelerator, resulting in nuclear fusion. The team had then hoped to introduce the atom to water and nitrogen to form simple molecules, but soon found it had already happened. ‘We had a bit of a surprise,’ says physical chemist Jennifer Pore, who led the project. ‘We were producing nobelium molecules even before we tried. The nobelium ions were reacting with trace contaminants of nitrogen and water in our gases.’
The results, confirmed by a mass spectrometer, included a variety of nobelium complexes containing hydroxide, water and dinitrogen ligands. While compounds containing heavier elements have likely been made, the short-lived nature of the isotopes – usually measured in seconds – means that definitive confirmation is not possible, and instead relies indirect measurements. This new experiment means nobelium is now the heaviest element with a definitively identified compound.
The experiment is part of a wider programme of research by Pore that aims to investigate one of the periodic table’s most enduring mysteries: which end of the lanthanide and actinide series of elements belong in group 3? ‘You can learn a lot of interesting things about these molecules,’ Pore explains. ‘You can learn about preferred oxidation states, and that has a good tie-in to periodic table placement. But gas-phase chemistry can give you direct information about electronic orbitals, and how accessible electron configurations are.’ While nobelium fits the trend for the actinides as expected, Pore is now planning to investigate the next elements in sequence, including lawrencium (element 103), rutherfordium (element 104) and dubnium (element 105).
Thomas Albrecht, an actinide chemist at the Colorado School of Mines, US, describes the work as an ‘important milestone in expanding our understanding of how chemistry evolves in the outer reaches of the periodic table’. ‘One can predict that these mass spectrometer measurements will lay to rest some of the confounding data that exists for the chemistry of the heaviest elements,’ he adds.
References
J L Pore et al, Nature, 2025, DOI: 10.1038/s41586-025-09342-y














No comments yet