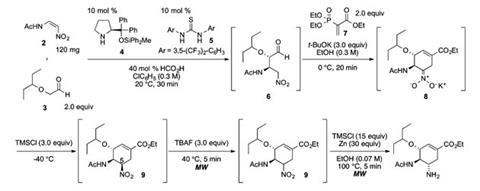
Production process showcases how organocatalysis can combine reactions in a single pot
Japanese chemists have smashed a synthetic speed record, cutting reaction time for making antiviral drug Tamiflu (oseltamivir) from over 30 hours to just one hour. ‘Imagine, after you get flu, you synthesise Tamiflu, and after 60 minutes the drug is ready,’ says Yujiro Hayashi from Tohoku University. ‘It would be great.’
Tamiflu, marketed by Swiss-based healthcare company Roche, has been plagued both by questions about its efficacy and challenges in its production. Its ring structure features groups around three adjacent chiral centres that are so difficult to assemble synthetically that Roche has instead exploited a semisynthetic route. That approach builds on shikimic acid, found in Chinese star anise flowers, now mostly made by bacterial fermentation.
The drug is therefore an icon for chemists flexing their synthetic muscles. Since 2008 Hayashi has used it to hone capabilities offered by an organocatalyst he had previously developed. The ‘Jørgensen–Hayashi catalyst’ is an organosilane molecule that controls chirality at carbon atoms joined via Michael reactions. Hayashi also sought to minimise changes between reaction vessels, as processing steps in-between are particularly time-consuming. Organocatalysts support this ‘pot economy’ concept as they’re less likely to interfere with subsequent reaction steps than more expensive organometallic alternatives.
From 2009–2013 Hayashi’s team went from three pots to one, but even then the process still took 57 hours. That’s partly because the nitroalkane and aldehyde joined by the Michael reaction aren’t very reactive, they realised, so Hayashi and his student Shin Ogasawara strove to speed the step up. ‘Combining my catalyst, acid and thiourea can greatly accelerate the reaction,’ Hayashi says.
However, the final reduction in the five step process generates equal amounts of both possible epimer products. This slows matters down as the chemists must separate them using chromatography, although Hayashi claims this is ‘not difficult’. The process yields 15% of the theoretical maximum final product, which is roughly 70% average yield for each step. Although Hayashi wants to improve this, he emphasises that speed reduces production costs. ‘Moreover, you do not need large stocks when the drug can be synthesised in a short time,’ he adds.
‘Throughput is the most crucial property in industrial synthesis,’ comments Svetlana Tsogoeva, from Friedrich-Alexander University in Erlangen, Germany. ‘This is accomplished in a very clever way through Hayashi’s work. The sequence of five steps in one pot can reduce waste, effort and costs.’ She adds that time economy might become considered more regularly in future syntheses.








No comments yet