MRI tag based on fluorine selectively 'switch on' in the presence of a target protein
Researchers in Japan have invented a new way to detect the presence of proteins in cells and tissues by magnetic resonance imaging. The technique involves using a nanocluster of fluorinated tags which ’light up’ in the presence of a specific protein but remain invisible in the protein’s absence.
Doctors and scientists are keen to image specific proteins in tissues and cells as a useful tool in diagnosing diseases as well as for basic scientific research. Conventional proton MRI can be used if an appropriate ’contrast agent’ can be attached to the protein. This usually entails constructing an antibody against the protein, which carries the contrast agent - typically based on metals such as gadolinium - to the target. However, because of the large amount of water in biological material it can be difficult to obtain a strong signal against signals from protons in the background.
19F gives a good MRI signal with no interfering background ’noise’ but relatively few systems have been developed to tag proteins with fluorinated compounds for MRI. Now, a team led Itaru Hamachi of Kyoto University has developed a fluorine-based label that remains cloaked from MRI in the absence of a target protein, but in its presence becomes visible.
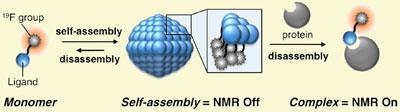
The probe consists of a hydrophobic chain, at one end of which is a hydrophilic F-containing group and at the other a ligand specific for the target protein. Because of the amphiphilic nature of the probe, it spontaneously aggregates into nanoparticles in an aqueous medium, with the fluorinated head ’buried’ within the particle. In this state the probe delivers only a weak MRI signal. However, when it meets its protein target, the ligand binds to the active site causing the nanoparticle to disintegrate into its constituent monomers, providing a sharp NMR peak typical of a small molecule, and thereby a clear MRI signal. In this way the signal is ’switched on’ only when the nanocluster meets the target protein.
The researchers successfully demonstrated their new technique with the enzyme carbonic anhydrase as a target protein. ’Based on this concept, one can flexibly design various "turn-on" type 19F probes for many applications,’ Hamachi says.
Philip Blower, an MRI expert at King’s College London in the UK, says, ’It is very clever. It is certainly a novel way to cause a change in signal strength in response to specific biomolecular processes, and these kinds of responses are sought after as "smart" contrast agents for medical imaging.’ Blower adds, however, that for the idea to be more generally useful, more understanding of how the particles stick together and the mechanism by which the protein binding causes them to dissociate will be needed.
Simon Hadlington
References
1






No comments yet