The promising anticancer compound nutlin-3 will be more widely available thanks to a straightforward new synthesis
The promising anticancer compound nutlin-3 is likely to become more widely available to researchers thanks to a new synthetic protocol developed by US chemists.
Nutlins, a group of compounds centred on a nitrogen-containing heterocycle, were discovered by scientists working for Hoffman-La Roche in 2004 and were found to inhibit a key interaction between two proteins involved in cancer pathways, with nutlin-3 the most potent of these.
The compound has attracted widespread interest but details of its synthesis are difficult to glean from the available literature - no full protocol has been published. The molecule has multiple chiral centres and synthesising the required stereoisomer is difficult.
Tyler Davis and Jeffrey Johnston at Vanderbilt University in Tennessee have used catalysts they developed to devise a straightforward synthesis of nutlin-3 that is highly selective for the required stereoisomer.
The key step in the synthesis is an aza-Henry, or nitro-Mannich, reaction involving the addition of a nitroalkane to an imine. Uncontrolled addition of the two substrates for the nutlin-3 reaction produces a total of four stereoisomers, whereas only one is effective.
Davis and Johnston had previously demonstrated that high stereoselectivity for aza-Henry reactions can be achieved by using electron-rich chiral bis(amidine) (BAM) catalysts. They screened a range of these compounds and identified one that promoted the reaction to give only the required nutlin stereoisomer.
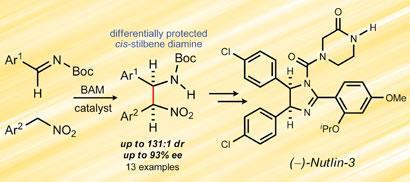
’We are not sure of the precise mechanism, but believe that the nitroalkane substrate forms a salt with the BAM catalyst by donating a proton,’ says Johnston. ’This complex in turn binds to the imine substrate, promoting the addition reaction.’
The chirality of the catalyst ensures that only certain faces of the substrates can come into contact. This geometrical constraint results in one stereoisomer product being favoured over the others.
’We think this is a chance to substantially increase access not only to nutlin-3 but also its derivatives,’ Johnston says. ’It is a relatively straightforward synthesis using standard techniques.’
Commenting on the work, Jim Anderson, an expert in stereoselective organic synthesis at University College London in the UK, says, ’The beauty of this synthesis lies in the recognition that the beta-nitro amine product from the nitro-Mannich or aza-Henry reaction has each amine essentially orthogonally protected. Asymmetry is efficiently provided by the development of the Johnston group’s bis-amidine catalysts. It’s a nice example of a very underused classic reaction that should be used more in synthesis now stereochemistry can be efficiently controlled.’
Simon Hadlington
Link to journal article
Catalytic, enantioselective synthesis of stilbene cis-diamines: A concise preparation of (-)-Nutlin-3, a potent p53/MDM2 inhibitorTyler A. Davis and Jeffrey N. Johnston,?Chem. Sci., 2011, 2, 1076DOI:10.1039/c1sc00061f






No comments yet