Short-lived reaction intermediates observed by x-ray in the pores of crystalline 'coordination networks'
Chemists in Japan have shown how it is possible to take sequential x-ray snapshots of chemical reactions taking place within molecular-sized ’reaction chambers’, capturing the crystal structures of short-lived reactive intermediates which are usually difficult or impossible to observe by other means. The technique should be widely applicable to a range of reactions, the researchers say, providing important new direct insights into reaction mechanisms.
A team led by Makoto Fujita of the University of Tokyo constructed a porous crystalline ’coordination network’ consisting of organic and inorganic components, and embedded the pores with an amine. They then took a crystal of the material and diffused acetaldehyde into it, while cooling it to 90K.
When the acetaldehyde meets the amine inside the pores it converts firstly to a hemiaminal intermediate before forming its product, a Schiff-base. Under normal conditions the hemiaminal is short-lived and difficult to observe. However, within the crystalline environment of the network’s pore the hemiaminal itself crystallises, and reducing the temperature ’freezes’ the crystal for long enough to enable x-ray analysis on its structure to be carried out. When the temperature is lifted, the reaction proceeds to its conclusion.
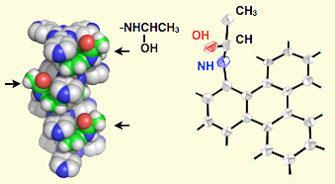
’We selected a very common standard reaction to demonstrate our concept,’ says Fujita. ’This method is applicable to other reactions whose mechanism or intermediate structures are unknown. Observing reaction intermediates clarifies reaction mechanisms, from which we can design reaction pathways, namely new reactions, for a family of related compounds.’
Commenting on the study, Jim Tucker, an expert in supramolecular chemistry at the University of Birmingham in the UK, says, ’The ability to identify and visualise an intermediate in a reaction using x-ray crystallography is clearly an exciting development. In this particular system, one of the reactants - the amine - is intercalated as part of the network but even so, one can envisage several reactions, including reversible processes, that can be followed in this way.’
Simon Hadlington
References
T Kawamichi et al, Nature, 2009, 461, 633 (DOI: 10.1038/nature08326)






No comments yet