A highly unusual multi-radical structure that has up to four unpaired electrons, yet remains stable, could find uses in batteries and computer memory
A quick experiment at the start of a PhD has resulted in a stable organic compound with four unpaired electrons. The researchers are now investigating this unusual structure for applications in batteries and data storage.
When Jonathan Barnes joined Fraser Stoddart’s group at Northwestern University in Evanston, US, the group were working on radical enhanced molecular recognition and trapping pairs of molecules in close contact. The team were using molecules held within a macrocycle and then removing two electrons to create a radical pair. Barnes looked at some of the other projects going on in the lab and wondered if something similar could be done with the group’s catenanes – interlocked chemical rings. ‘A lot of people said it wasn’t possible because logically it doesn’t make sense to have this much charge so close together,’ says Barnes. ‘It was a pie-in-the-sky kind of goal I was reaching for but it turned out actually that it worked, and it worked on the first try.’
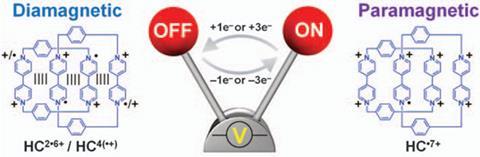
These catenanes are perfect for this kind of chemistry as they’re made of BIPY units, bipyridiniums that are able to form radical cations when subjected to one electron reduction. The team then successfully stripped electrons one-by-one from the interlocked rings getting down to a stable structure with four unpaired electrons. ‘A lot of the radical chemistry described in the Science paper has been well known since the 1930s,’ explains Barnes. ‘It’s pretty old chemistry, but not many people picked it up after that.’
The key to the stability of the new radical compound is the mechanical bond that links the two macrocycles, forcing the charged species to remain close. And that proximity means that the molecule never oxidises to a fully charged species but stops at the paramagnetic species •7+. That, says Barnes, is because the molecule is trying to minimise the charge in the centre where the two catenanes link. ‘The charged units have no choice but to interact with one another,’ explains Barnes, so it holds on to that remaining electron to reduce the charge repulsion.
But while the •7+ species is paramagnetic, all the other charged species are diamagnetic, as the electrons spins pair. Using cyclic voltammetry it is possible to quickly switch between the paramagnetic and diamagnetic states by adding and removing electrons. And it is this simple switching that could be the key to a potential application of the material: memory storage.
First off though, Barnes and Stoddart are working with a team in Korea to investigate the use of the material in lithium ion batteries. Batteries, explain Barnes, need materials that accept electrons pretty well ‘and that’s what the catenane does’.
A Prasanna de Silva of Queens University Belfast, UK, describes the work as a fine paper that ‘will make the career of young Jonathan Barnes’. The novelty of Barnes’ material, he adds, is that the high density of positive charges makes it resist becoming fully charged in air. ‘Since the paramagnetic state can be reversibly converted to several diamagnetic states,’ says de Silva, confirming Barnes, ‘the current molecular magnets will likely find information storage applications since they are easily switchable, long-lived and metal-free.’







No comments yet