It is 50 years since Karl Ziegler and Giulio Natta won the Nobel prize for their work on polymers. Mike Sutton traces the story of their discoveries
During world war two it was widely believed that Britain’s fighter pilots were fed carrots to improve their night vision. Vitamin A is necessary for healthy eyes, and carrots do contain one of its biochemical precursors, beta-carotene. But this rumour was a cover story – the success of RAF night-fighters was really due to their top-secret radar equipment.
Until the late 1930s, the size and weight of radar apparatus made fitting it into fighter planes impossible. But this situation, and the course of the war, was altered by two British innovations. One was the cavity magnetron, a compact device for generating electromagnetic waves. The other was a new electrical insulator – polythene.
Polythene was difficult to make. At first, only its remarkable insulating properties made it worth bothering with. Yet soon after the war ended it became a mass-produced commodity, with innumerable industrial and domestic uses. This revolution grew out of the discoveries made by Karl Ziegler (1898–1973) and Giulio Natta (1903–1979), joint winners of the 1963 Nobel prize for chemistry.
Intriguing chemical invention
A series of fortunate accidents paved the way for them. In 1898, the German chemist Hans von Pechman had noticed that heating diazomethane – a hazardous operation few were eager to repeat – produced an inert white solid, initially known as ‘polymethylene’.
But its potential remained unsuspected until scientists at Imperial Chemical Industries (ICI) re-encountered it in 1933. Like most British industry, ICI was hit by the depression which followed the 1929 stockmarket crash. Yet instead of imposing an austerity regime, the company invested in more research. Part of that push was 50 experiments at high pressures, which generated one exploitable result. Reginald Gibson and Eric Fawcett heated benzaldehyde and ethylene (ethene) to 170°C at 1900 atmospheres, hoping to synthesise ethyl phenyl ketone. Instead, they got an intriguing waxy solid.
It was soon identified as a polymer of ethene, with a molecular weight of around 4000. In 1935, ICI chemists Edmond Williams, Michael Perrin and John Paton produced larger amounts by heating and compressing ethene alone – or so they thought (later, they found that their ethene supply contained traces of oxygen, which were necessary to catalyse the polymerisation). Various names for the product were mooted and ‘polythene’ was eventually chosen.

ICI began manufacturing it as an insulator for underwater telephone and telegraph cables. But when the threat of war pushed Britain’s radar programme into overdrive, polythene also proved to be an exceptionally efficient insulator for cables carrying high frequency signals. The radar pioneer Robert Watson-Watt later said ‘The availability of polythene transformed the design, production, installation and maintenance problems of airborne radar from the almost insoluble to the comfortably manageable.’
After the war, ICI’s process remained strategically valuable, but the extreme conditions it required made the product relatively expensive. Polythene’s low melting-point and limited mechanical strength were further handicaps. In 1951 Robert Banks and Paul Hogan of the Phillips Petroleum Company in the US developed a catalytic process that was more efficient, though still using high pressure. The crucial breakthrough came in 1953, when Karl Ziegler introduced catalysts which polymerised ethene under far milder conditions.
Ziegler’s catalysts
Born in 1898 in Germany, the son of a Lutheran pastor, Ziegler entered Marburg University in 1916. Although his studies were interrupted by war service, he completed his PhD in 1920, and in 1922 he married Maria Kurtz. They raised two talented children, and many of Karl’s students remembered Maria fondly as a motherly ‘Frau Professor’. After holding senior appointments at Heidelberg and Halle universities, Ziegler was appointed director of the Max Planck Institute for Coal Research at Mülheim in 1943.
At that time, Mülheim was exposed to heavy Allied bombing, being situated in the Ruhr valley industrial region, so Ziegler went there only when necessary. His contract allowed him to conduct research outside the field of coal utilisation, and initially he kept his professorship, and his family home, in Halle. But in the spring of 1945, the Zieglers left Halle in a US Army vehicle, shortly before Russian troops occupied the city.
In due course the Mülheim Institute was refurbished (with financial assistance from the Marshall Plan) and Karl, now its full-time chief, played a significant part in reconstructing West Germany’s chemical industry. When the German Chemical Society was re-formed in 1949, he became its president.
Its potential was recognised by the great organic chemist Robert Robinson
Organometallic compounds had been one of Ziegler’s major interests for many years, and in the post-war era at Mülheim he became heavily involved with aluminium alkyls. Though useful intermediaries in organic syntheses, they needed careful handling, being spontaneously inflammable when exposed to air. He hoped they would assist him in converting ethene – a relatively cheap by-product of coal-gas manufacture – into more valuable hydrocarbons. The results greatly exceeded his expectations.
The process worked because triethyl aluminium can combine with ethene (and higher alkenes) under pressure. These intermediates then yield progressively larger hydrocarbon molecules while regenerating the catalyst.
Industrial chemists were unimpressed by this result, however, arguing that the mixed hydrocarbons it yielded would be prohibitively expensive to separate. But its potential was recognised by the great organic chemist and 1947 Nobel laureate Robert Robinson. In addition to his position at Oxford, he was then a consultant for Petrochemicals Limited, which later became British licensees for Ziegler’s process. Another early enthusiast was Giulio Natta, the Italian chemist who eventually shared the Nobel award with Ziegler.
Natta’s collaboration
Natta, born in Italy in 1903, was a lawyer’s son. Having studied maths at the University of Genoa, he completed a PhD in chemical engineering at Milan Polytechnic in 1924 and taught there until 1933. After holding professorships at Pavia, Rome and Turin, he returned to Milan in 1938 as director of the polytechnic’s Industrial Chemical Research Centre, where he spent the rest of his career. In 1935 he married Rosita Beati, a professor of literature at Milan University. They had two children.
While visiting Germany in 1952, Natta heard Ziegler lecture and invited him to Milan for discussions with the Montecatini chemical company. It was agreed that the professors would collaborate on further investigations, while Montecatini (who already supported Natta’s own polymer research) would become the Italian licensees for Zeigler’s process. Three Italian research students – funded by Montecatini – joined Ziegler at Mülheim in February 1953. Soon afterwards, they alerted Natta to a major new development there.
Ziegler reasoned that if one metal deactivated the catalyst, another might encourage it
When one series of attempts at polymerising ethene consistently failed to produce molecules larger than butene, Ziegler suspected that traces of nickel from the walls of the high-pressure reaction vessel were inhibiting the formation of higher polymers. He reasoned that if one metal deactivated the catalyst another might encourage it, and his co-workers soon found several which did.
Polythene was not their original goal, but in November 1953 they made it – at only a few atmospheres pressure – by adding a zirconium compound to the triethyl aluminium catalyst. Early in 1954, using a titanium co-catalyst, they produced polythene at atmospheric pressure. Heinz Martin rushed into Ziegler’s office with a sample, exclaiming ‘Es geht in Glas!’ (‘It goes in glass!’)
Perfect polythene – and beyond
The operation is relatively straightforward. In the absence of air, ethene is bubbled through an inert solvent containing aluminium triethyl and titanium tetrachloride, and polythene gradually precipitates. At the molecular level, the process is more intricate. Unstable complexes – involving alkyl aluminiums, titanium chlorides and alkenes – interact in a multi-phase environment, with some reactions apparently occurring on the surface of microcrystals of titanium trichloride.
Ziegler’s low-pressure polythene was mechanically stronger than ICI’s high-pressure version, and had a higher melting point. This was partly due to its greater molecular weight. But x-ray diffraction revealed another reason – its straight-chain molecules were packed together more compactly than the heavily branched chains of ICI’s polythene.
Probing the structures of big molecules with x-ray and electron diffraction techniques had been Natta’s speciality for many years, and his expertise contributed significantly to the partnership. But Natta also wanted to help Montecatini recover their investment in his research. After hearing about the Mülheim group’s success with polythene, he began secretly investigating a possibility which they had ignored.
Ziegler’s early attempts to polymerise propylene (propene) had failed. He assumed this reaction was impossible, asserting authoritatively that ‘Es geht nicht’ to Natta and others. Consequently, Ziegler prioritised the search for new catalysts ahead of experiments with different monomers, and polypropylene was not synthesised at Mülheim until June 1954. On applying for a patent, Ziegler discovered that Natta had beaten him to it.
The Italians had produced polypropylene in March 1954, using what Natto tactfully described as a ‘Ziegler catalyst’. Despite the compliment Ziegler was seriously offended, believing that Natta had broken the terms of their agreement. The two remained estranged for years, although the breach was healed in time for them to receive their Nobel awards together.
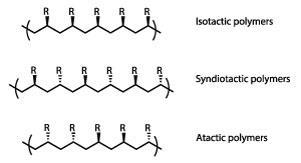
Natta’s extensive x-ray and electron diffraction studies showed that altering the catalysts and reaction conditions could yield polymers with widely varying molecular structures and physical properties. Molecular chains could be made longer or shorter, straight or branched, according to need – with significant commercial consequences.
In the case of polypropylene he found that the orientation of its methyl groups was crucial, and he gave its stereoisomers distinctive names which were originally suggested by his wife. ‘Atactic’ polypropylene (with randomly oriented side-groups) is an amorphous rubbery material of limited utility. In contrast, the ‘syndiotactic’ variant (with methyl groups on alternate sides) coils into a regular helical structure, giving fibres with considerable mechanical strength.
A plastic world

Polypropylene eventually found its own niche in the market, but it was polythene which transformed the plastics industry in the 1950s. A multitude of products – from washing-up bowls to water supply pipes – were soon being made from it. Unfortunately, after a few months’ use many of them developed cracks, and only another happy accident saved the industry from serious embarrassment.
The cause of this problem was insufficient cohesion between the unbranched molecular chains that Zeigler’s reaction produced. The answer was to add small quantities of other hydrocarbons to the ethene feedstock, creating a limited number of side-chains which made the polymer molecules lock together more firmly. However, until this improved material became available, manufacturers remained burdened with large stocks of apparently unsaleable plastic.

Fortunately for them, the Wham-O Toy Company launched its ‘Hula Hoop’ in 1958. Cheaply made out of extruded polythene tubing, it was imaginatively marketed as both a toy and an exercise aid for slimmers. Unexpectedly high demand for it soon absorbed the stocks of ‘old’ polythene (which was robust enough to survive this ephemeral craze). Plastics manufacturers were relieved, polythene’s progress resumed, and Ziegler and Natta duly received their well-deserved honours.
Ziegler’s personal patents made him extremely wealthy. In 1969 he retired in comfortable circumstances, having donated 40 million Deutschemarks – around £4.2 million at the time – to a fund for chemical research, and was able to travel the world viewing solar eclipses. Natta’s retirement was not so happy. In 1956 he was diagnosed with Parkinson’s disease, but despite its debilitating impact he was able – with support from his family – to accept his Nobel prize in person.
Throughout the half-century which has elapsed since that award, the plastics industry has continued producing polythene profitably. Meanwhile, radical innovations like metallocene catalysts and fluidised-bed reactors have transformed production methods and a multitude of products – ranging from high-tech surgical implants to low-cost footwear – have made life better for billions of people worldwide. Nevertheless, polythene now confronts us with a massive problem.
Products made from this almost indestructible substance need to be disposed of responsibly when they are broken or no longer wanted. At present, much of the world’s superfluous polythene is simply dumped, causing serious environmental damage. Attempts to reduce its impact, whether by legislation or education, have so far made only limited progress. The ingenuity of chemists gave us polythene – we may need to draw on that ingenuity again to deal with the unintended consequences of their gift.
Mike Sutton is a visiting fellow in the department of humanities at Northumbria University, UK
Further reading
H R Allcock, F W Lampe and J E Monk, Contemporary polymer chemistry, Pearson Education, 2003
ICI Plastics Division, Landmarks of the plastics industry, Kynoch Press, 1962
F M McMillan, The chain straighteners, Macmillan, 1979
G Natta, Nobel lecture, http://bit.ly/19ibAYe
K Ziegler, Nobel lecture, http://bit.ly/1blOdV7
R H Olley, The story of polythene http://bit.ly/1bl67ns

















No comments yet