Mike Sutton tells the tale of John Kendrew and his work towards the unveiling of protein structures
Between 1939 and 1945 many British scientists were actively engaged in wartime projects. But while their professional expertise could be crucial at key moments, their routine workload was often mainly administrative. In Charles Percy (C P) Snow’s novel The new men, a fictional scientist probably spoke for numerous real-life ones by declaring that when peace returned he would ‘put my in-tray on top of my out-tray and go back to something worth doing’.
Yet for a number of returnees to academia, finding ‘something worth doing’ was problematic, because the enforced career break had made them rethink their objectives and seek exciting new challenges. John Kendrew, born 100 years ago this month, was one such adventurer. When the war began, he was a physical chemist just starting his postgraduate research on reaction kinetics. When it concluded he wanted to investigate biologically important molecules using x-ray crystallography. However, as he later recalled in his 1962 Nobel lecture: ‘My own almost total ignorance of this method was fortunate, in that it concealed from me the extent to which contemporary x-ray crystallographic techniques fell short of what was needed to solve the structures of molecules containing thousands of atoms; it was indeed a case of ignorance being bliss.’
To remedy this ignorance, Kendrew went to the University of Cambridge’s Cavendish Laboratory, supported by an ICI research fellowship. Lawrence Bragg and Max Perutz were pursuing similar enquiries there, and Francis Crick and James Watson would later begin investigating DNA alongside them. In this intellectual hothouse Kendrew’s career blossomed. But by then he had already come a long way.
The early years
John Cowdery Kendrew was born on 24 March 1917 in Oxford, where his father, a reader in climatology at the university, was responsible for his upbringing. (His mother, an art historian, spent most of her career in Florence.) At the University of Cambridge, Kendrew studied physics, chemistry, biochemistry and mathematics in part one of his degree, specialised in chemistry for part two, and gained first class honours in 1939. By February 1940. he was working for the Air Ministry – initially on radar, but soon with a wider brief.
Britain’s new radar-based air defence system needed an efficient flow of information up and down the chain of command, and to organise better communication networks a revolutionary method of data analysis known as operational research was developed. It proved to have much wider applications and Kendrew became one of many scientists using it on urgent logistical and strategic problems.
He met Linus Pauling who had tried x-ray diffraction on proteins in the 1930s
This work took Kendrew around the globe, leading to two encounters which influenced his post-war career. In Sri Lanka he talked with John Desmond Bernal, then a senior government scientist but previously an eminent x-ray crystallographer. And on a military mission to the US he met the chemist Linus Pauling – also currently involved with war work – who had tried x-ray diffraction on proteins in the 1930s, though with limited success.
Kendrew ended the war as an honorary wing commander in the RAF, and could have remained in government service afterwards. Instead he returned to Cambridge, but not to his pre-war doctoral project. Partly as a result of his conversations with Bernal and Pauling, he chose a fresh line of research under the guidance of Perutz.
Perutz was an Austrian chemist of Jewish ancestry who began studying proteins at Cambridge in the 1930s, and remained there after political developments made it dangerous for him to return home. In 1939 he was interned, along with thousands of other ‘enemy aliens’, but later released to work on secret British military projects.
After the war ended Perutz returned to Cambridge and with funding from the Medical Research Council established a molecular biology group, which Kendrew joined. Having completed his doctorate, Kendrew continued working alongside Perutz, and in 1962 shared the Nobel prize in chemistry with him. But although their research was highly innovative, it rested on foundations already laid by others.
Standing on the shoulders of giants
In 1912 the German physicist Max von Laue (assisted by Paul Knipping and Walter Friedrich) demonstrated that orderly ranks of atoms in crystals could produce interference patterns in a beam of x-rays – just as more widely-spaced regular structures generate analogous patterns by interacting with the longer waves of visible light. A year later, in Britain, Lawrence and William Bragg applied this technique to show that the atoms in a crystal of common salt had a cubical structure, resembling the external shape of the crystal itself.
Binary compounds like sodium chloride offered obvious clues to the internal organisation of their crystals. Even with more complex molecules like naphthalene, physical and chemical data could narrow the range of possible structures ahead of attempts to interpret the x-ray results. But exploring molecules that were biologically significant (and much larger) proved extremely difficult.
Success often depended on the investigator’s ability to form mental images of 3D structures
Among those who accepted this challenge during the interwar years were Bernal at Cambridge, and his former research student Dorothy Hodgkin at Oxford. Their first problem was preparing usable crystals of these delicate (and often very uncooperative) substances. When a crystal was finally obtained, monochromatic x-rays were directed at it, and its diffraction pattern recorded on a photographic plate. This operation was repeated again and again, each time with the crystal at a different angle.
Every spot in the resulting photographs recorded the diversion of part of an x-ray beam by the crystal’s atoms. Each spot’s intensity and position indicated the strength of that portion of the ray and the angle through which it had been diverted. At first, analysis of these figures seldom produced more than a general outline of a molecule’s structure, though it might support (or eliminate) structures already proposed on chemical grounds.
Two mathematical tools (Fourier synthesis and the Patterson function) were helpful in this endeavour, but getting a result could still take months – even years – of effort. Success often depended on the investigator’s ability to form mental images of 3D structures which were compatible with the diffraction data, and with the substance’s known chemical properties.
Hodgkin was particularly adept at this. In 1942, she produced the first complete structure for a biologically significant molecule (cholesterol, molecular weight approximately 385). Another breakthrough followed in 1945, when she used a mechanical computer, programmed with punched cards, to facilitate the calculations which led her to the structure of penicillin (molecular weight approximately 330). But it was not until electronic computers became available a decade later that Perutz and Kendrew’s success with much larger molecules became possible.
Uncovering life’s building blocks
Haemoglobin’s role as the blood’s oxygen carrier made it a prime target for investigation. Earlier work had shown that this molecule included four iron-containing haem units and four protein chains. But its sheer size (its molecular weight is over 64,000) handicapped Perutz’s pursuit of its structure, which was still ongoing when Kendrew completed his doctorate – a comparative study of adult and foetal sheep haemoglobin – in 1949.
Perutz finally achieved his goal in 1959, but meanwhile Kendrew had focussed on myoglobin, which stores oxygen inside muscle tissue and has a molecular weight of approximately 17,000. He sourced it from whale meat – the muscles of diving mammals are exceptionally rich in myoglobin – but struggled to prepare crystals large enough, and stable enough, for x-ray analysis.
A crucial factor in Kendrew’s success was his deployment of a powerful but technically challenging crystallographic technique – isomorphous replacement. Bernal, Hodgkin and others had obtained valuable data about biologically significant molecules by comparing the x-ray diffraction patterns generated by two crystals of subtly different composition. The differences can reveal important information about the structure of the molecules making up the crystals. Generally, one of the pair was a naturally occurring substance, and the other was synthesised from it by replacing one of its atoms with a heavier atom from the same group, or by adding a heavier atom to the mother liquor during crystallisation. Perutz refined this technique, and Kendrew eventually developed it further – but first, he had to find an element that could be substituted, atom for atom, into the myoglobin molecule, and then persuade the new compound to form usable crystals. This was not an easy task.
In his Nobel lecture, Kendrew explained that he and his assistants tried many times to insert heavier atoms into the myoglobin molecule, at sites which theory predicted would be suitable. When these efforts failed, they were thrown back to a more empirical approach, crystallising myoglobin in the presence of metallic ions and then seeing whether any changes in the x-ray pattern could be detected. Further analysis then determined whether or not substitution had taken place at a single site, as desired. In the absence of any sound foundation in theory, it was necessary to examine several hundred possible ligands before two or three suitable ones were found.
In his Nobel lecture, Kendrew explained that he and his assistants tried many times to insert heavier atoms into the myoglobin molecule, at sites which theory predicted would be suitable. When these efforts failed, they were thrown back to a more empirical approach, crystallising myoglobin in the presence of metallic ions and then seeing whether any changes in the x-ray pattern could be detected. Further analysis then determined whether or not substitution had taken place at a single site, as desired. In the absence of any sound foundation in theory, it was necessary to examine several hundred possible ligands before two or three suitable ones were found.
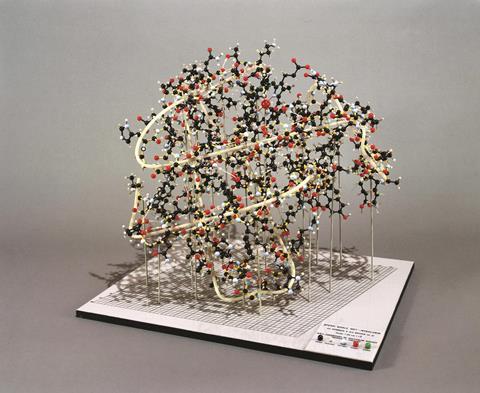
Analysing many x-ray diffraction pictures of natural and modified myoglobin eventually allowed him to begin mapping the molecule’s 3D structure. This required the processing of enormous amounts of data. The complete diffraction pattern of a myoglobin crystal, synthesised from many separate observations, was composed of at least 25,000 individual spots (known as reflections). In 1955, when the team began to convert this data into a 3D map of the molecule no computers existed fast enough to calculate Fourier syntheses containing so many terms, explained Kendrew.
In 1957, figures from 400 reflections went into Edsac I – one of the very few electronic computers then in existence. It generated an electron density map of myoglobin with a resolution of 6Å. Although the molecule’s structure was irregular and asymmetrical, its general outline was reasonably clear.
A far more detailed picture emerged from the analysis of 9,600 reflections in 1959. Processing this mass of data pushed current technology to the limit, but fortunately two colleagues (Ulrich Arndt and David Phillips) developed an automatic diffractometer which was introduced part-way through the task. Readings from it were recorded directly on to punched tape and fed into a faster computer, Edsac II.
With a resolution of 2Å, what had previously appeared as rod-like sections of myoglobin’s polypeptide chain were now shown to be helical in form, resembling structures that Pauling had already detected in other proteins. Using this data, Kendrew and his co-workers were finally able to map myoglobin’s convoluted 3D structure, locating its functional groups (and three-quarters of its individual atoms) with considerable precision. (In the 1960s they further improved the resolution to 1.4Å, by processing the data from 25,000 reflections on larger computers such as the IBM 7090.)
Kendrew’s unravelling of the myoglobin structure – together with Perutz’s success with haemoglobin, and the parallel advances of Watson, Crick, Rosalind Franklin and Maurice Wilkins with nucleic acids – helped to launch a revolution in biology. The building blocks of life and their assembly instructions were finally becoming accessible.
Later life
For many years Kendrew remained heavily involved in this rapidly developing field. Until 1987, he was editor-in-chief of the Journal of Molecular Biology (which he founded in 1959), and later he became chief editor of the 1997 Encyclopaedia of molecular biology. A fellow of the Royal Society from 1960, he received its Royal Medal in 1965 and numerous other honours thereafter, including a knighthood in 1974.
Kendrew’s influence extended well beyond the boundaries of academia. His 1964 BBC television series The thread of life helped to make recent advances in molecular biology – and their momentous implications for the future of humanity – accessible to the general public. He also provided expert advice to successive British governments, first as a member of the Council for Scientific Policy and then as chairman of the Defence Scientific Advisory Committee, Meanwhile he encouraged international scientific cooperation, travelling widely to lobby for his own specialism (in fluent French, German and Italian). When the European Molecular Biology Laboratory (EMBL) opened at Heidelberg in 1975, he became its first director general.
Of Kendrew’s personal life, little need be said here. In 1948 he married Mary Jarvie, a widow who later qualified as a medical practitioner. Their marriage was dissolved in 1956. From 1947 to 1975 he was a fellow of Peterhouse College in Cambridge, but after retiring from the EMBL in 1982 he migrated to Oxford, serving as president of St John’s College until 1987. Kendrew had a long association with the UN, and on his death in 1997 much of his estate went to a fund supporting students from the developing world.











No comments yet