With the international year of crystallography upon us, Clare Sansom celebrates this important discipline
Much of our collective attention during 2014 is bound to be focused on commemorating the start of the first world war. That ‘war to end all wars’ undoubtedly had a profound effect on world history. But the second decade of the twentieth century should also be famous for a series of discoveries that laid the path for a completely new scientific discipline: crystallography, the study of atomic and molecular structures through crystals. It has transformed chemistry and materials science, and paved the way for modern molecular biology, and to celebrate, the United Nations has declared 2014 to be the International Year of Crystallography.

It can be said, perhaps simplistically, that crystallography was invented by the father-and-son team of William Henry and William Lawrence (known as Lawrence) Bragg, at the Universities of Leeds and Cambridge in the UK. The Braggs, however, did not aim to found a new discipline or even to investigate the atomic properties of matter. They were more interested in solving a problem that had been puzzling the cleverest physicists in the world for almost two decades. X-rays had been discovered by Wilhelm Röntgen in Germany in 1895, but their very name (the unknown X) reveals their controversial nature. Were they particles or waves?
In 1912, another German physicist, Max von Laue, showed for the first time that these x-rays were scattered (or diffracted) when shone at a crystal and that this produced a pattern on a film, lending credit to the wave theory. The Braggs heard of von Laue’s work in a letter to William from his colleague Lars Vegard, the Norwegian physicist. William was initially convinced that x-rays consisted of particles and set out with his son to prove that this was so. Lawrence became convinced that they were waves, however, and subsequently persuaded his father of this.
Lawrence Bragg realised that the patterns could be explained by the theory that the x-rays were reflected from planes of atoms in the crystal and interfered with each other. ‘We understand now, of course, that electromagnetic radiation, including x-rays, can be thought of as both particles and waves, and therefore that both Braggs were right,’ explains Mike Glazer, emeritus professor of physics at Oxford University and an expert in the early history of crystallography.
Lawrence’s law
Lawrence Bragg’s first results were presented to the Cambridge Philosophical Society in November 1912 and published in the society’s proceedings at the beginning of 1913 in a paper titled ‘The diffraction of short electro-magnetic waves by a crystal’. Lawrence, newly graduated from Cambridge at the age of 22, was the first to understand that the x-rays initially used by Laue were ‘white’ – that is, of multiple wavelengths – and how this affected the pattern of spots.
Bragg’s paper contains the first formulation of one of the most widely recognised of all the laws of physics. Bragg’s law, as generations of students have learned it, relates the spacing between planes of atoms in a crystal (d), the wavelength of an incoming beam of x-rays (?) and the diffraction angle (?) through the simple equation n ? = 2d sin ?. Therefore, if you know the wavelength and can measure the diffraction angles, you can determine the plane spacing and thus – through some complex mathematics – the geometry of the crystal lattice concerned.
The diffraction patterns the Braggs obtained from diamond could explain for the first time both its hardness and its sparkle
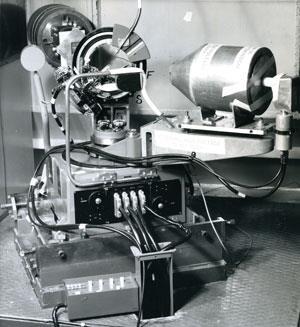
Following this, Lawrence worked with his father to make precise measurements of the x-ray spots. The equipment they used looks extremely simple to 21st century eyes. It was designed by William and built by his instrument maker CH Jenkinson in a workshop at Leeds, and it had an almost Heath-Robinson appearance (see The Braggs’ spectrometer). ‘The ionisation spectrometer, as the Braggs called it, enabled a beam of single wavelength x-rays to pass through a rotatable crystal into an ionisation chamber where the diffracted waves were detected,’ says Glazer. ‘This is the ancestor of all the diffraction equipment we use today.’ The first structure solved using this equipment was that of sodium chloride – common salt – and this too was published in 1913.
The Braggs continued to work together on the structures of simple substances until the war intervened and Lawrence was sent to France. One of the first structures they studied was a sample of diamond borrowed from the mineralogy collection in Cambridge. As the Braggs’ namesake (and believed to be distant relative), broadcaster Melvyn Bragg, explained in a radio programme celebrating the centenary ‘The diffraction patterns the Braggs obtained from diamond could explain for the first time both its hardness and its sparkle.’
Röntgen was awarded the first ever Nobel prize in physics in 1901 for his discovery of x-rays; von Laue was awarded the same prize in 1914 and the Braggs’ Nobel followed a year later. Their prize citation was simply ‘for the use of x-rays to determine crystal structure’. The 25-year-old Lawrence Bragg received news of his prize while serving in the trenches, shortly after that of his brother Robert’s death at Gallipoli. He remains the youngest ever recipient of any Nobel prize.
A maturing field
These prizes were the first of a long list. The International Union of Crystallography maintains a list of Nobels awarded for ‘achievements directly related to, or involving the use of, crystallography’. There are now 29 of these, and the latest year with no crystallography-related Nobel was 2008. Even the 2013 chemistry prize, awarded to Martin Karplus, Michael Levitt and Arieh Warshel, appears on the list: their discipline of computational chemistry would be impossible without structural knowledge obtained through crystallography.

Many events have been planned to celebrate the work of the Braggs and their co-workers, and the International Year. With the help of the Royal Institution, Glazer planned and mounted a wide-ranging exhibition, ‘The Two Braggs’ that was held alongside the 28th European Crystallographic Meeting in Warwick in August 2013; it was opened by the UK government’s chief scientific advisor, Mark Walport, and attended by several of the Braggs’ descendants. ‘The exhibits we were able to display included many original models of molecules; replica Nobel medals; the letter from Lars Vegard to William Bragg that started him thinking; and four of the six ionisation spectrometers known to have been used by the Braggs,’ says Glazer. ‘And one of the other two seems to have disappeared in Canada.’
It is not surprising that the molecules and structures studied by the Braggs were some of the simplest known: their main aim was to develop the experimental techniques and associated mathematics. Once that was established, other scientists, many working alongside the Braggs, began to use the technique to determine the structures of slightly more complex molecules. Both William and Lawrence Bragg were pioneers in supporting talented women scientists. Kathleen Lonsdale is perhaps the best known of the women who worked with the older Bragg as students and later as colleagues.
‘Until Lonsdale published the molecular structure of hexamethylbenzene in 1929, no-one had proved that the benzene ring was flat,’ says chemical crystallographer Judith Howard of the University of Durham, UK. Lonsdale worked on benzene derivatives, rather than on benzene itself, because benzene is a liquid at room temperature, and it has only hydrogen substituents on its ring: both these features of the molecule complicate its analysis by crystallography.
The years leading up to the second world war saw numerous advances in the field, but two stand out in particular. In Cambridge in 1934, John Desmond (JD) Bernal and his student Dorothy Crowfoot, later Hodgkin, obtained the first x-ray diffraction patterns from crystals of a protein. A year later at the Massachusetts Institute of Technology in the US, Lindo Patterson developed a function that greatly simplified the mathematics involved in structure determination. Both Bernal and Patterson had worked with William Bragg at the Royal Institution in London, and both have been considered to be among the most brilliant scientists never to win a Nobel prize.
But solving the structures of even relatively small molecules remained difficult in the 1960s and beyond, as Howard remembers. Her first paper, published in 1966 while she was an undergraduate at the University of Bristol, describes the synthesis and structures of some metal carbonyl clusters. ‘When I started in crystallography, data was still collected on photographs,’ she recalls. ‘One of the biggest breakthroughs in my early career came when it became possible to scan and digitise these images’.
Howard also remembers the frustrations of trying to solve structures with the earliest computers, and the length of time it all took. ‘When I was a PhD student, a typical thesis contained the structures of perhaps three small molecules’, she says. ‘It’s now possible to solve as many in a single morning.’ And now that a structure can be solved in a few hours, many other types of experiment have become possible. Physical chemists can now see exactly how a material responds to changes in temperature or pressure, or to light, by solving the same structure repeatedly under different conditions.
Increasing intensity
Any x-ray diffraction experiment requires a beam of x-rays, and the power and precision of that beam affects the type of experiments that can be performed. The most powerful x-ray beams that are used today arise from synchrotron radiation sources; these were discovered in the 1950s but not exploited as tools for crystallographers for several decades. Elizabeth Shotton, industrial liaison manager at the UK’s synchrotron light source, Diamond, explains: ‘When particle physicists first observed that charged particles emit electromagnetic radiation when accelerated radially, they naturally focused on the fact that for high-energy physics experiments this loss of energy was a nuisance; they only gradually came to realise that it could be exploited in other scientific applications.’
The first dedicated synchrotrons built primarily as useful sources of radiation were constructed in the 1980s. The UK’s first such synchrotron, the SRS at Daresbury in Cheshire, opened for business in 1980 and closed 28 years later, the year after its successor Diamond opened in Harwell in Oxfordshire.
Many of today’s synchrotrons look like huge doughnuts from the air; Diamond’s ring is over half a kilometre in diameter. A synchrotron ‘doughnut’ is not entirely circular, however: it is polygonal, with electromagnetic radiation generated whenever the beam of particles – almost always electrons – bends. The intense radiation, ranging from infra-red to high-energy or ‘hard’ x-rays, is captured by beamlines that are designed to optimise its properties for the experiments required.
‘You can think of a synchrotron beamline as a lab, each producing beams of synchrotron radiation with slightly different power and wavelength, and offering scientists different experimental techniques,’ says Shotton. Diamond, currently the fifth largest synchrotron in the world, has 23 operational beamlines with another 10 under construction. The facility is currently used by about 3,000 scientists and currently has over 60 industrial customers carrying out R&D work that requires access to world-class instrumentation and scientific expertise.
Sandy Blake, a crystallographer from the University of Nottingham in the UK, is using both the small molecule single crystal diffraction high-intensity hard x-ray beamline and the powder diffraction beamline to determine the structures and study the properties of metal–organic frameworks (MOFs): novel materials that are able to store gases. ‘Some MOFs are very porous, and the structure of the pores determines how efficiently they can store hydrogen and greenhouse gases such as CO2 and SO2. X-ray crystallography is the only technique that can determine this,’ says Blake.

Powder diffraction is the main technique for determining the structure of materials that do not necessarily form large single crystals. There are synchrotron beamlines optimised for this technique, in which high brightness x-ray beams interact with micro-crystalline samples. Synchrotron powder diffraction enables structures to be determined at exceptionally high precision, and beamlines equipped with advanced detectors can collect powder diffraction patterns very fast.
Chiu Tang is the principal beamline scientist working on the high-resolution powder diffraction beamline at Diamond. ‘We can program our robot to collect diffraction data from 200 powder samples for high-throughput experiments (a few seconds per pattern), with the option of using non-contact devices for low and high temperature measurements,’ he says. ‘We can also run experiments at temperatures ranging from close to absolute zero to 2000K using cryogenic apparatus and furnaces.’
Beyond x-rays
Electrons, neutrons and more exotic fundamental particles also obey Bragg’s law, with their wavelength determined by their speed of travel, so they too can be used for diffraction. ISIS, which is located on the same site in Harwell as Diamond, generates fast-moving beams of neutrons for diffraction by firing protons at tungsten targets. The wavelengths of these neutrons range from 0.05 to 14Å, which is similar to those of x-rays used in crystallography.
Neutron diffraction can be used to pinpoint the exact positions of hydrogen molecules bound to MOFs
David Keen
David Keen is a leading researcher at ISIS and also the current president of the British Crystallographic Association. ‘The most fundamental difference between neutron and x-ray diffraction is that x-rays interact with electrons, and neutrons with nuclei. So neutron diffraction, unlike x-ray diffraction, can see hydrogen atoms almost as clearly as heavy metal atoms such as lead’, he says. ‘Kathleen Lonsdale would have been fascinated to see how diffuse neutron diffraction experiments have grown from her x-ray work to understand molecular packing in materials such as benzil.’
The structure of benzene was first solved in 1958 using x-ray diffraction, and this molecule continues to interest crystallographers: the most precise benzene structure to date was determined by a group led by Howard using both x-ray and neutron diffraction, and published in 2002. And there are many other examples of synergy between X-ray and neutron diffraction techniques. ‘Sandy Blake collaborates with groups working with neutron diffraction, as this technique can be used to pinpoint the exact positions of hydrogen molecules bound to MOFs,’ says Keen. The total number of ‘small’ (and medium-sized) molecules with 3D structures obtained using x-ray or neutron diffraction and stored in the Cambridge Structural Database is now well over half a million.
At a hundred years old, crystallography is still a relatively young discipline, but it has already radically transformed many other scientific disciplines and, through them, the world we inhabit today. But astute readers will no doubt have noticed that there is something missing from this article. That, of course, is macromolecular crystallography: from the structure of DNA to those of complex molecular machines, the history of structural biology deserves a feature to itself – which will follow later in the year.
Clare Sansom is a science writer based in London, UK












No comments yet