A sudden change in the properties of a drug as a new polymorph appears can be highly damaging for pharma firms. The industry now appears to be in control of the situation
A sudden change in the properties of a drug as a new polymorph appears can be highly damaging for pharma firms. The industry now appears to be in control of the situation

The pharmaceutical industry faces a deadly and swift saboteur. This attacker hits unexpectedly and comes under the title ’polymorphism’: when compounds adopt more than one crystal structure or polymorph in the solid state. A new polymorph can be catastrophic; overnight a drug manufactured for market can become less soluble and less bioavailable. Although a compound’s polymorphs are chemically identical, their physical properties can be tremendously different.
’Polymorphism is completely unpredictable,’ says Chris Frampton, chief scientific officer of SAFC- Pharmorphix, a Cambridge, UK-based company which investigates solid state properties for the pharmaceutical and biotechnology industries. ’The worst that can happen for a pharmaceutical company is if a new polymorph suddenly appears in the temperature and humidity conditions of a blister pack when a compound is actually on the market.’
Polymorphism was first discovered in 1821 by the German chemist Eilhard Mitscherlich and is a widespread phenomenon in the chemical world. Polymorphs differ in their outer form (habit) as well as their inner structure (form). For example, polymorphs of the amino acid glycine exist either as prisms or as a pencil shape, while paracetamol crystals may appear as prisms or monoliths (see fig, p68). Polymorphs may differ in melting point, solubility, density, hardness, and in optical and electrical properties. In industrial terms, these differences may affect manufacturing and processing properties, shelf life, texture, stability against air and light, ability to be compressed into a tablet, and so on.
The first polymorph to form may be metastable and liable to convert to a different, more stable, form over time or under different conditions of re-synthesis. Or the most stable polymorph at room temperature may become metastable at another temperature; transitions between polymorphs may occur as the temperature is raised or lowered - a crucial consideration when shipping a pharmaceutical or food product. The US Food and Drug Administration (FDA) thinks these issues important enough to require companies to register the polymorphic forms of any new drug they make.
It is likely that all small organic molecules have the potential for polymorphism, because there are so many ways in which molecules can be packed within a crystal, mainly through different ways of arranging hydrogen bonds between neighbouring molecules.
’It is a matter of finding the right conditions for a polymorph to form,’ explains Frampton. Sometimes these conditions may be so extreme - very high pressure, for instance - that the polymorph’s existence is likely to remain undiscovered indefinitely. A drug or food product is made many times over; any changes in synthesis conditions, however subtle, may encourage the emergence of new polymorphic forms. ’We want to ensure the crystallisation step yields the same polymorph each time,’ says Frampton.
Roland Boese, of the University of Duisberg-Essen, Germany, quotes estimates that 30 to 50 per cent of pharmaceutically important compounds exhibit polymorphism. If hydrates and solvates are included, then perhaps as many as 90 per cent of compounds will exist in more than one crystalline form.
Industrial cost
In industry, the existence of polymorphs is best discovered sooner rather than later, as US pharmaceutical firm Abbott Laboratories learnt to its cost. In 1998 the company found that it could no longer make the original form of its HIV drug ritonavir because a second polymorph had taken the place of the first during manufacture. Abbott scientists had discovered that the drug was starting to fail dissolution tests and precipitating out of capsules. Investigation showed the existence of polymorph II (needles), which was more soluble and less stable than polymorph I (rods). Form II soon spread and form I could no longer be synthesised. Form II’s decreased solubility could have affected the drug’s bioavailability and Abbott was forced to withdraw ritonavir from the market for a time while it figured out how to get the original polymorph back; its loss in sales was compounded by extra development costs. Abbott scientists finally found a way to get back, consistently, to form I while also discovering a way to manufacture form II exclusively. This involved a carefully controlled reflux and cooling cycle, keeping the solutions and reactor as free as possible from form II contamination.
Even long-established drugs can be hit by polymorphism. In 2005, a team of researchers led by Michael Zaworotko of the University of South Florida, US, claimed to have discovered and made a new polymorph of aspirin: form II (see fig, p70). Then, last year, a team from Denmark, Germany and India, led by Boese, claimed to show that the crystals analysed by the US team were in fact an intergrowth of two polymorphs: form I and form II. Pure form II is elusive, they say.
Structure screening
Ritonavir served as a wake-up call for the pharmaceutical industry, which became more interested in exploring the solid state of drug candidates at an earlier stage. Previously, companies had been reluctant to part with precious milligrams of a new compound for these studies - especially when they might not even go ahead with developing the compound.
Now polymorph screening is a common procedure, using robots to carry out hundreds or thousands of crystallisation experiments using different solvents or solvent mixtures and different cooling and evaporation rates. Boese, one of the international team that analysed aspirin’s form II, adds that some scientists still discover polymorphs using a more empirical approach. ’There is no general rule, but some more or less intuitive guidelines do exist, sometimes kept secret by experimentalists.’
A stable polymorph is obviously a potentially good choice for a pharmaceutical company to take into further development. But stability is not the only consideration. ’A metastable form may have better dissolution properties, which then improves the bioavailability,’ says Boese. ’But the metastable form may then transform into the stable form, which is then less soluble. This was the case with ritonavir.’ On the other hand, as Frampton points out, a compound can be too soluble - it may take in water during storage.
Once a desired polymorph has been discovered, the next step is to learn how to obtain it reliably - and stop less desired polymorphs from forming. The crystallisation process is not well understood but involves nuclei forming within a supersaturated solution of the compound. The nuclei act as templates for the formation of further crystals within the solution. Without nucleation, the supersaturated solution may not crystallise for a long time, which is why chemists sometimes try to form nuclei by scratching the side of the vessel containing the solution with a glass rod. Tiny crystals of the desired polymorph - if they exist - can act as nuclei or seeds for crystallising that polymorph. But seeding doesn’t always work as planned - if seeds of an undesired polymorph are present, even in a very small amount, they will tend to seed that polymorph, instead of the wanted one. Impurities can also act as seeds. ’The purer a compound is the less likely templating is. As a compound becomes purer, there could be a switch to a different polymorph,’ says Bill Jones, head of the chemistry department at Cambridge University, UK.
Crystallisation need not be so unpredictable. Recent research from Mike Ward’s group at the University of Minnesota, US, suggests that the process can sometimes be controlled using synthetic porous polymers as a crystallisation surface. The pores may limit the size of nucleus that can form and therefore drive the formation of a particular polymorph. Working with the compound ROY, which has several polymorphs (see box, above), the Minnesota team found that only one of them formed within the pores.
How many polymorphs?
Many compounds such as sucrose and naphthalene appear to have only one polymorphic form. At the other end of the spectrum is 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile - also known as ROY (red orange yellow) because of its seven solvent-free polymorphs. These occur as red or yellow prisms, orange plates or needles, yellow needles, orange-red plates, and red plates.
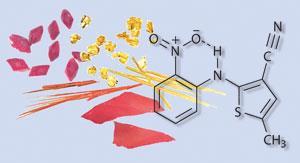
ROY is an intermediate in the synthesis of the antipsychotic drug olanzapine and its polymorphism was discovered by Lian Yu, now of the University of Wisconsin-Madison, US, and coworkers in 2000. Two other forms of ROY, both yellow crystals, were discovered in 2005. ROY holds the Cambridge Structural Database record for the most polymorphs of any solved crystal structure. A survey carried out by Yu in 2000 found 321 polymorphic systems of which 291 had two polymorphs, 27 had three, three had four, and none had five or more.
Patentable polymorphs
Although polymorphism may sound like a trying challenge for the pharmaceutical industry, it does have its positive side. Polymorphs are patentable. If an early screen reveals the existence of two or more polymorphs, a company can patent them all, choose to develop one and then, when it comes to the end of its patent life, go back and develop one of the others to extend the life of the drug - a process that Frampton calls ’evergreening’ or life cycle extension.
The issue of the ownership, identity and characterisation of pharmaceutical polymorphs has led to several lengthy court battles. For instance, ranitidine hydrochloride - the anti-ulcer drug Zantac - has long enjoyed blockbuster status. Glaxo (now GlaxoSmithKline or GSK) discovered a second polymorph of ranitidine hydrochloride early in
the drug’s development and took out a patent. The second form proved easier to manufacture
and became the drug’s active ingredient.
When the patent on the first form expired, generic companies were naturally keen to make their own versions. But the very existence of the second form, still under patent, made it hard for competitors to make the first form without contamination. This scientific problem bought GSK valuable time by delaying the appearance of generic versions of Zantac.
Lengthy court battles have also been waged over polymorph ownership for the antidepressant paroxetine hydrochloride (Seroxat), the antibiotic cefadroxil, and terazosin hydrochloride, a drug for prostate problems.
Predicting the unpredictable
In future, computational methods may be able to take some of the unpredictability out of polymorphism. Jones’s Cambridge group has already made important computational steps. Maleic acid, an important salt-forming agent in the pharmaceutical industry, is a key example. Since maleic acid’s crystallisation in 1881, it had been believed that there was just one polymorphic form but Jones recently discovered a second, in the form of colourless plate-like crystals, after co-crystallisation with caffeine. His team was able to use a computational approach to predict the existence of this second form - and others.
Meanwhile, a team from University College London (UCL), under its e-Materials project and led by Sally Price, used computational techniques to predict a new polymorph of the Alzheimer’s drug piracetam - polymorph IV. Colin Pulham from Edinburgh University’s high-pressure group had already discovered a new form of the drug in the lab and challenged Price to tell him its structure. ’We were correct in the prediction we sent him,’ she says. Early work as part of the e-Materials project had also predicted aspirin’s form II. ’It’s exciting to see a new polymorph appear,’ says Price. ’This is such a fun area to be in - when we calculate that more polymorphs are feasible than are already known, either new polymorphs are found, or we need to work out why not.’

The UCL work is part of a polymorphism project, Control and prediction of the organic solid state, funded by the Research Councils UK.
Computational methods are still in relative infancy. ’They cannot be applied to very complex active pharmaceutical ingredients at present, says Frampton. ’But we are aware of the possibility and are working towards it with various academic groups. Ideally, you would be able to take a two dimensional structure and predict a molecule’s physical and chemical properties in the solid state. We are nowhere close to this.’ Price agrees that computation could not have saved Abbott from the ritonavir crisis; the molecule is too big and contains lots of unusual functional groups. ’But in a few years I think we could tackle a molecule like this,’ she predicts.
It would seem that a multi-pronged approach to spotting new polymorphs is the key, combining computational chemistry with analytical lab techniques (see box, below). Given polymorphism’s potential to destroy pharmaceutical sales targets, it is well worth companies staying or becoming vigilant. After all, it’s not all negative - discovering a new polymorph can be the key to scientific and financial success.
Susan Aldridge is a freelance science writer based in London, UK
How to spot a polymorph
Single-crystal and powder x-ray diffraction are the gold standard for determining how molecules are packed in the crystal; advances in this technology mean that ever smaller crystals can be used. Differential scanning calorimetry (DSC) allows the detailed study of the crystal’s behaviour as it is heated and cooled, to determine the stability range of different polymorphs and to identify any changes from one polymorph to another. ’DSC is a good way of finding new polymorphic forms,’ comments Chris Frampton, chief scientific officer of SAFC-Pharmorphix, UK. Infrared, Raman, and nuclear magnetic resonance spectroscopy (NMR) are used because the different hydrogen bonding patterns in polymorphs give rise to different spectra; added to these are solid state NMR and terahertz (far infrared) spectroscopy (see Chemistry World, March 2007, p52), emerging technologies which will further refine the characterisation of polymorphs. Meanwhile, gravimetric vapour sorption determines how much water a compound absorbs and whether it forms hydrates. Finally, a compound may be exposed to what Frampton calls the ’tanning salon’ which simulates the effect of long periods of sunlight exposure on a crystal form. These data are important for determining a polymorph’s possible behaviour during shipping and storage.
Further Reading
- A D Bond et al, Angew. Chem. Int. Ed., 2007, 46, 615
- P Vishweshwat et al, J. Am. Chem. Soc., 2005, 127, 16802
- Ed: Rolf Hilfiker. Polymorphism in the pharmaceutical industry, Wiley VCH, 2006
- S Chen et al, J. Am. Chem. Soc., 2005, 127, 9881
Companies
- Pharmorphix is based in Cambridge, UK, and provides solid-form research services to the international pharmaceutical and biotechnology industries. It uses a range of techniques to predict polymorphs.
- TeraView, Cambridge, UK, provides terahertz spectroscopic systems for polymorphism analysis. Terahertz spectroscopy can, for example, differentiate between different hydrate forms.
- Bruker, US, sells a range of machines that can be used to hunt for polymorphs. Bruker AXS, for example, offers x-ray solutions, while Bruker Optics sells Fourier transform infrared, near infrared and Raman spectrometers. Bruker has developed a one-off prototype hybrid x-ray and Raman machine for Pharmorphix (above).
- Anachem, Luton, UK, sells a ’parallel chemistry product’ (ReactArraySolo) which contains a series of mini reaction vessels with different temperature zones. These allow temperature profiles to be set up to drive the formation of polymorphic forms.
- PANalytical, the Netherlands, supplies analytical instrumentation and software for x-ray diffraction and x-ray fluorescence spectrometry. The company is part of Spectris, Egham, UK.
- Zinsser Analytic, Germany, sells an automated liquid and powder handling platform for polymorphism screening studies. The platform, Crissy, can be used to analyse a series of crystals using x-ray powder diffraction.
- Solvias, Switzerland, offers a polymorphism service which covers polymorphism screening, crystal purity and kinetic stability, crystallisation optimisation and monitoring, and a controlled scale-up of the crystallisation process.






No comments yet