With N2 O emissions up 40% in four decades, scientists are searching for answers. Anthony King looks at potential solutions to keep fertiliser nitrogen in the soil
- Nitrous oxide (N2O) is a potent greenhouse gas and the leading ozone-depleting substance, with emissions rising 40% from 1980 to 2020, mainly due to agricultural use of nitrogen-rich fertilisers and manure. It has a long atmospheric lifetime and is 273 times more warming than CO2 over 100 years.
- Microbial processes in soil are central to N2O generation, especially under low-oxygen conditions where microbes switch to denitrification, releasing N2O as an intermediate. Soil conditions, fertiliser type, and timing significantly influence emissions.
- Farm-level mitigation strategies include better fertiliser timing, use of protected urea and nitrification inhibitors, and innovative approaches like adding crushed silicate rock to raise soil pH and reduce emissions. However, cost and uncertain yield benefits limit widespread adoption.
- Broader systemic changes are needed, including improved nitrogen use efficiency, industrial abatement technologies, and policy shifts involving fertiliser companies and food systems. While challenges remain, increased attention to N2O offers co-benefits for climate, air and water quality.
This summary was generated by AI and checked by a human editor
The buildup of nitrous oxide in the atmosphere is accelerating faster than predicted. This matters because N2O is the third most important greenhouse gas and the foremost destroyer of ozone in the upper atmosphere. These were conclusions from a UN report released in November 2024, which dubbed the gas a super pollutant. A major driver is agricultural nitrogen.
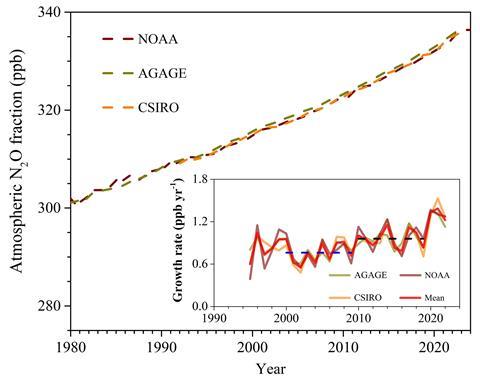
Emissions of N2O from human activities went up 40% in the four decades from 1980 to 2020, according to a recent estimate. Most of this stems from adding nitrogen-rich synthetic fertilisers and manure, with microbes using nitrogen compounds for metabolism. Levels of N2O have risen from 280ppb before industrialisation to 336ppb in 2024. ‘About 10% of the warming we’ve seen since the industrial revolution can be tied to nitrous oxide,’ says David Kanter, environmental scientist at New York University in the US. The UN reported concluded that ambitious action could cut the equivalent of up to 235 billion tonnes of CO2 emissions by 2100, which is around six years of current carbon dioxide emissions from fossil fuel burning.
Once a molecule of N2O is released from the soil surface, it spends on average 120 years in the atmosphere and contributes around 273 times more to warming than CO2 per mass over a period of 100 years. ‘We refer to the hydroxyl radical that oxidises many organic compounds as the cleaning agent of the lower atmosphere, but it doesn’t react with nitrous oxide,’ says Rona Thompson, atmospheric scientist at Norwegian research institute NILU in Kjeller. N2O is impervious to oxidation until it drifts up to the stratosphere, where high-energy light particles break it down mostly into dinitrogen. Its persistence means that its warming impact accumulates and lasts a long time.
A small fraction of N2O breaks down to nitric oxide (NO) and nitrogen dioxide, which can repeatedly destroy ozone (O3) in the upper atmosphere. The ozone layer filters out ultraviolet light and was protected by the Montreal Protocol in 1987 that phased out ozone-killing industrial chemicals. At the time, N2O seemed a minor player and was known to be generated in natural processes, but it is now viewed as a serious threat to stratospheric ozone. ‘It is the largest remaining threat to the ozone layer by far and could undo a significant part of the success of the Montreal Protocol,’ warns Kanter.
How soil microbes drive emissions
The rising N2O emissions result mainly from biologically useful nitrogen being added to soil, a boom created by the Haber–Bosch process in 1910 and industrial-scale ammonia (NH3) production. We can reduce how much nitrogen is applied. But it is crucial to understand how soil conditions and microbes influence how N2O is generated.
As a rule of thumb, about one percent of nitrogen fertiliser added to soil enters the atmosphere as nitrous oxide. In between there’s a series of chemical and biological transformations of nitrogen. ‘It comes out of microbes because they fiddle with the redox state of the nitrogen molecule,’ says Lars Bakken, microbiologist in the nitrogen group at the Norwegian University of Life Sciences. Microbes kick off by oxidising ammonia to a short-lived molecule (hydroxylamine, NH2 OH), itself oxidised to nitrite (NO2–), generating a small amount of energy. Specialised bacteria convert nitrite to nitrate (NO3–) in the presence of oxygen, yielding only a small amount of energy. A small quantity of N2O is generated as a byproduct.
The nitrate itself is susceptible to being lost from farm soils. Ammonium as a cation sticks to negatively charged clay, but nitrate is negatively charged and is notoriously leaky. ‘Nitrate itself is super mobile, which is great for plant uptake, but it can be leached quite quickly,’ says Wolfgang Wanek, a biogeochemist at the University of Vienna in Austria. Nitrate can pollute rivers and lakes and then be converted into N2O. This nitrification path is not how most N2O is generated on farms, however.
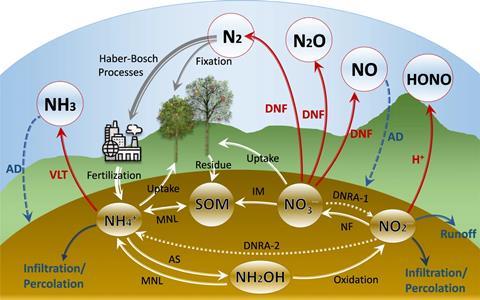
Instead, some soil microbes, which use denitrification if oxygen runs short, are responsible. ‘We breathe in oxygen and breathe out CO2. These denitrifiers breathe in nitrate and breathe out nitrogen gases,’ explains Peter Groffman, a soil ecologist at the City University of New York, US. Oxygen boasts plenty of capacity to accept electrons, making it the first choice for respiration. In its absence, soil microbes make do with compounds such as nitrate, the first domino in a line of nitrogen electron acceptors in the soil. The sequence: nitrate to nitrite to nitric oxide (NO) to N2O and finally to dinitrogen (N2). Microbial species can specialise in one or more of these steps. A major product of this anaerobic respiration is harmless N2, but N2O generation can be substantial under certain conditions.
Farm-level solutions show promise
A first step in reducing emissions is to add the right amount of fertiliser at the right time – when the plant is growing. Adding nitrogen to soil chock full of nitrate will result in more losses, including nitrous oxide. Timing matters. The worst case scenario is to fertilise wet soil when rain is due, which encourages nitrate runoff, and more nitrous oxide generation because a lack of oxygen encourages microbes to switch to dentrification. After fertilising or irrigation, short-lived pulses of N2O from soil can account for 90% of emissions.
One challenge is that farmers are incentivised not to scrimp on nitrogen fertiliser and get shortchanged on valuable yields. Too much can damage crops, however, and EU regulations limit nitrogen additions. Europe has also been successful in boosting nitrogen use efficiency: the proportion of nitrogen that goes into the crop. Tillage and leaving land left unplanted after a harvest can both encourage emissions of N2O. ‘You can get a lot of emissions from fallow land,’ asserts Thompson. Cover crops help.
Matching a fertiliser to a specific soil type reduces waste, though growers must weigh up costs. ‘Urea is the cheapest type of nitrogen fertiliser you can buy,’ says Karl Richards, head of the climate centre at Teagasc, a farm advisory organisation in Ireland. Urease are ubiquitous soil enzymes and chop urea into two molecules of ammonia and one of carbon dioxide in the form of ammonium (NH4+) and bicarbonate. At higher temperatures and higher acidity, ammonia gas can form and disperse. ‘Ammonia typically gets deposited within one or two kilometres from where its emitted. When it comes back onto soil it can release nitrous oxide,’ says Richards. In Ireland, Teagasc has advised farmers to switch to urea fertiliser with urease inhibitors (protected urea) to reduce ammonia release. This is a strategy to save farmers money and reduce nitrous oxide emissions. Almost no Irish farmers used such ‘protected urea’ five years ago, whereas today it is half of all urea, Richards says.
Another option is to add nitrification inhibitors that interfere with the ammonia monooxygenase enzyme that catalyses the oxidation of ammonia to hydroxylamine, the first step in nitrification. They are not popular with farmers due to cost. ‘There’s two chemical nitrification inhibitors on the market in Denmark but they are not used much,’ says Carsten Suhr Jacobsen, microbial ecologist at Aarhus University in Denmark.
Inhibitors reduce N2O emissions, but it is uncertain whether they improve crop yields, giving little incentive to farmers. Their effectiveness varies with soil conditions, rainfall, temperature and crops. One study found that an inhibitor (3,4-dimethylpyrazole-phosphate, DMMP) reduced N2 O emissions from sandy soil but not loamy soils and from horticulture but not farming. In northern Denmark, Jacobsen is running experiments on barley fields to monitor the impact of nitrification inhibitors on groundwater, soil structure and microbes. ‘We plan to measure impacts using new DNA sequencing technology looking at genes,’ he explains. The results should reveal whether farmers should be incentivised to use such nitrification inhibitors to reduce N2O. There are sceptics. ‘I don’t like it. It’s putting a chemical into the soil,’ says Bakken.
Another option is to coat fertiliser particles with organic or inorganic polymers to slow nitrogen release. Lots of formulations of slow-release fertiliser have been described. ‘Some of those protective coats are used on the horticulture side,’ says Richards, but the added costs means they are not economical for most farmers.
Other innovative options are pursued. One strategy is to add crushed silicate rocks such as basalt to farmland, which produces bicarbonate ions. This draws down CO2 but can also cut emissions of other greenhouse gases. ‘When you apply basalt, it increases the soil pH, leading to a decrease in N2O emissions,’ says Maria Val Martin, an atmospheric chemist who reported on the effect of such enhanced rock plus fertilisers with nitrification inhibitors. The effect is partly because nitrification and denitrification are not as favoured at higher soil pH. Research or policy interventions to encourage this practice are ongoing in some countries.
Val Martin says crushed silicate rock can supply some micronutrients and trace elements; she estimates that 20 tonnes per hectare could have a lasting impact on emissions of N2O but also of ammonia. ‘We are seeing in fields a reduction of 18 to 20% in N2O emissions, which is significant,’ says Val Martin. In field experiments, she is testing how frequently the treatment would be needed. It may substitute for the addition of lime, often required because fertilisers can acidify soils.
Microbial interventions offer quick wins
The loss of nitrous oxide from farms mostly comes down to unseen microbes that switch to nitrogen-containing compounds when oxygen runs low. This happens when soil is wet or when plentiful organic matter and a microbial boom consume available oxygen. ‘These organisms respire oxygen 95% of the time,’ says Bakken, ‘but they can switch to reducing nitrate, nitrate, nitric acids or N2O as electron receptors.’ This can be happening here and there in pockets of soil.
Individual microbes may harbour enzymes to reap energy from one or more of these steps. ‘Three compounds – nitrite, NO and N2O – are free intermediates, meaning that they are released and reabsorbed,’ says Bakken. The degree to which each is released into the soil depends on the activity of each of the four enzymes in the chain and which microbes with which enzymes are plentiful.
Bakken’s lab is especially interested in microbes with N2O reductase, as it is the only enzyme that can reduce the N2O to N2 – which makes up 79% of our atmosphere. ‘If the activity of this enzyme is very high, then N2O is absorbed from the environment and reduced to N2,’ says Bakken. Either gas is a loss of nitrogen for a farmer, but dinitrogen is harmless while N2O is a super pollutant.
Bakken believes that deliberately manipulating soil microorganisms holds great potential for boosting the conversion of N2O to N2 in soils. His group introduced a Cloacibacterium strain that can only respire N2O, not the other compounds in the denitrification chain, in a field experiment with digestate from a wastewater treatment plant. The idea would be for farmers to be incentivised to inoculate straw with this bacteria and dig it into the field; the bacteria would convert N2O to N2 for a few weeks after fertilisation, before succumbing to competition pressures from other soil microbes.
Measuring nitrogen is the world’s greatest contamination problem
The nitrous oxide reductase enzyme can be a weak link in supressing emissions of N2O. If nitrate concentrations in the soil are high, the microbes will continue to use this as an electron acceptor instead of nitrous oxide reductase, resulting in emission of N2O. ‘It’s a finicky enzyme,’ says Groffman. ‘It sits on the exterior of the cell and is subject to environmental conditions.’ It can be deactivated by more acidic soil conditions, making it unavailable for catalysing reduction of N2O to N2. ‘Even trace amounts of oxygen in the soil can affect the enzyme,’ adds Groffman. It is also a copper-rich enzyme and so requires this metal. It has been reported that inadequate copper supplies for the enzyme can encourage N2O emissions.
If denitrification runs to completion, N2 is the end product. This is the case in at least 50%, perhaps 80% of the time, says Wanek. Calculating how much N2 and N2O is emitted is enormously challenging. First, dinitrogen is difficult to measure because it makes up almost 80% of the air above soil. Measuring how much comes out from soil ‘is the world’s greatest contamination problem’, says Groffman. You must collect soil and then conduct technically demanding lab experiments in a helium–oxygen atmosphere or with stable isotopes. Wanek reckons there are just a few hundred measurements of N2 emissions from soils, compared to thousands for N2O.
The challenge with N2O is variability. Two chambers over soil next to one another can give wildly different readings. In Sweden, Bakken’s team uses robots on wheels to randomly take readings in a field. Another option is to use analysers placed on towers above fields. Atmospheric chemist Laura Cardenos at Rothamsted Research in the UK is analysing N2O emissions for government inventories at North Wyke in Devon. An analyser capable of detecting this gas costs around £180,000.
Tracking what happens to nitrogen fertiliser is a huge issue because a single atom of nitrogen can inflict a cascade of problems. It can be released as ammonia, an air pollutant, and touch down and get converted into nitrate, which may run into waterways. If enough nitrate runs into rivers or lakes, algal blooms can consume so much oxygen that fish die. Nitrate above certain levels in drinking water is a human health issue. It may also artificially fertilise and damage natural ecosystems. This water-borne nitrate can be released as nitrous oxide, which contributes to warming and ozone destruction.
Other benefits of nitrogen management
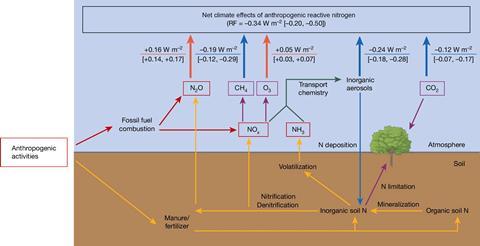
It is possible though to view this as a glass half full, nevertheless. ‘If you do manage nitrogen better, you get a whole host of co-benefits,’ says Kanter. There have been advances. ‘Industrial sources were low-hanging fruit,’ says Thompson. Almost all adipic acid plants in Europe and the US have stopped emissions. The nitric acid industry is now the largest source, supplied for the manufacture of fertilisers, munitions and adipic acid (for synthetic fibres and foam). Just one-quarter of the approximately 500 plants worldwide are fitted with abatement technology. This is according to the Nitric Acid Climate Action Group, which pushes for the phasing out of N2O emissions from these plants. Industrial sources account for around 5% of anthropogenic emissions.
Agriculture represents 75% of human emissions. About 70% of that is from fertiliser and the remainder from manure. There remain systems where the nitrogen use efficiency is poor and nitrous oxide emissions can be cut. ‘We do not need to consume globally as much nitrogen as we do to produce the amount of food we do,’ says Kanter. There have been advances. Chinese farmers had a reputation for adding too much nitrogen fertiliser but this situation has improved, according to Hanqin Tian, an earth system scientist at Boston College, US. He led a global budget calculation that showed the EU, Japan and South Korea reducing their anthropogenic emissions over the past four decades, especially from the chemical industry in the 1990s.
In the last five years, policymakers have paid more attention to nitrogen
Some countries still subsidise fertiliser, but most farmers were incentivised to economise after a precipitous rise in prices following the Russian invasion of Ukraine. Yet incentives pull farmers the other way. ‘The risk is that limiting nitrogen fertiliser can impact crop optimal yields and reduce farm profitability,’ says Richards.
They are constrained. ‘If you optimise all management related to a product before it leaves the farm, you could reduce emission by maybe 30%,’ says Cardenas. Some such as Kanter say that governments often put too much focus on farmers alone: fertiliser companies, supermarket and multinational food companies can play a role. Kanter suggests that fertiliser companies might be mandated to sell a proportion of fertiliser with specified emission standards, for example. Reducing meat consumption in some countries would also help lower emissions.
The rise of N2O emissions linked to agriculture is especially vexing because it is tied to food security. ‘In temperate regions it is difficult to grow the amount of food we need without nitrogen,’ says Cardenas. Some areas such as sub-Saharan Africa do not use enough fertiliser, according to a Food and Agricultural Organisation report on nitrogen. With the world population expected to approach 10 billion by 2050, we will need to grow more food. ‘We shouldn’t be overly optimistic about how much we can reduce nitrous oxide emission linked to fertiliser,’ warns Groffman.
It is not all doom and gloom. Paying more attention to nitrous oxide emissions will reap rewards in climate change and ozone and simultaneously improve water and air quality. Broader actions such as a drive to reduce nitrogen waste by 50% by 2030 automatically reduce nitrous oxide emissions. The issue is coming into focus. ‘In the last five years, policymakers have paid more attention to nitrogen. The last penny to drop has been nitrous oxide,’ says Kanter. ‘Even last 18 months has been transformative.’
Anthony King is a science writer based in Dublin, Ireland


















No comments yet