The final piece of the jigsaw puzzle of how actinides bond with ligands through phi bonds has been identified, with its control via oxidation states paving the way for advances in f-block catalysis and quantum computing.
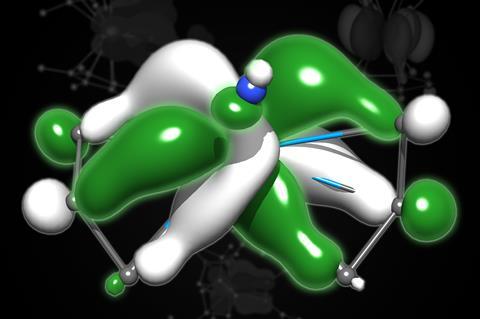
The actinide series includes the heaviest elements that occur naturally on Earth, and how they bond with ligands is essential to understand, both in terms of fundamental chemistry and in solving problems in the nuclear industry. However, their radioactivity and the complexity of working with these elements makes it difficult to confirm theoretical predictions.
The gargantuan size of these atoms also means they behave differently. Bonds are described based on the symmetry of their atomic orbitals; sigma bonds have no ‘nodal planes’, pi bonds have one and delta bonds, found in the transition metals, have two. The f-orbitals, such as those found in the actinides, have three nodal planes, forming rare, and still mysterious, phi bonds.
To help expand our understanding of the far end of the periodic table, a group of researchers led by Ping Yang at Los Alamos National Laboratory, US, used theoretical modelling to predict how these phi bonds behave. Building on previous work and knowledge of actinide structures, the team looked at actinide–ligand bonding across the entire series, and three different oxidation states, to see how these phi-interactions occurred. The team’s results showed that, simply by varying the oxidation state of the actinide, they could change the strength of phi bonding’s ‘head-to-head’ interaction: essentially providing an easy way to tweak how the actinide behaves.
This discovery could allow researchers to fine-tune actinide bonding, with potentially huge implications for the entire f-block, explains Yang. ‘This control enables the rational design of ligands with enhanced selectivity for actinides over lanthanides, particularly challenging for minor actinides like americium and curium, offering a powerful strategy to improve separation efficiency in nuclear fuel recycling.’
The group’s findings could also inform the design of better redox-active catalysts, and even control of electronic states, allowing f-block elements and their high angular momentum to be used in quantum computing, she suggests.
Conrad Goodwin, an actinide researcher at the University of Manchester, UK, praised the work as ‘a trove of data, which I am sure will be extremely valuable for the community’, likening it to the contributions made by former American Chemical Society president Bruce Bursten in terms of potential impact. ‘It’s a beautiful piece of fundamental theoretical actinide science,’ he adds. ‘This type of theoretical investigation is hugely important as it provides real, testable experimental targets for the synthetic community to explore.’
References
MJ Beltran-Leiva, ER Batista and P Yang, JACS Au, 2025, 5, 1746 (DOI: 10.1021/jacsau.4c01277)














No comments yet