Scientists tackle concerns over debated technique
A research group in the US has successfully simplified a crystallographic technique that scientists, bar those behind the original technique, had struggled to get to grips with.1
In 2013, Makoto Fujita’s group at the University of Tokyo in Japan unveiled a powerful tool that gave scientists the ability to analyse oils and liquids by x-ray crystallography, a seemingly impossible task. Upon incorporating these compounds into crystalline hosts, their molecules became ordered, and the compounds became susceptible to diffraction. Thus, armed with fresh insight, the method has provided the absolute structures of compounds where we’d previously been left in the dark.2
After the initial report,3 the method got off to a slow start, with Fujita admitting that the initial crystallography was not up to scratch.4 The technique has come a long way since, but it still has a way to go if it’s to fully convince the crystallographic community of its value. Now, Jon Clardy’s group at Harvard University is keen to do just that: ‘Crystallographers have generally expressed serious reservations about the method. We set out to put Fujita’s method on firmer footing,’ explains team member Timothy Ramadhar.
The crystalline sponge most-used thus far is a metal-organic framework based on zinc-iodide units; unfortunately, the technique’s main hurdle is that this framework contributes to the crystallographic data, masking the information available on the guest molecules. Since electrons interact directly with x-rays, the Harvard team decided to substitute the electron-rich iodine atoms for lighter bromine and chlorine atoms to reduce the overall host contribution. What they found came as a surprise – not only were the guests comparatively more visible, but the smallest repeating unit for each crystal was just a quarter of the size of that for the iodine framework. This means that where weeks of computational time were previously required, this was reduced to a matter of hours.
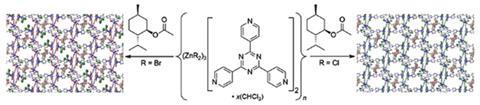
This approach does not solve all of the crystalline sponge method’s problems. ‘It should however facilitate structure refinement, especially with respect to reliably locating the guest molecules in the electron density map,’ comments Peter Müller, a crystallography expert from the Massachusetts Institute of Technology in the US.
Whilst the team will continue in their quest to improve the crystallography in the method, Ramadhar is convinced that the more sponges the merrier: ‘A suite of crystalline sponges must be developed to handle molecules with various properties and functional groups, the ultimate goal being to cover chemical space as widely as possible, while maintaining high crystallographic rigor.’










No comments yet