
A new technique for reducing nitrate ions in polluted wastewater has been developed by researchers in China and the US. The work, which does not rely on toxic or precious metals, works with water containing realistic concentrations of nitrate, so the researchers believe it could provide a viable alternative to the Haber–Bosch process for producing ammonia.
Ammonia is a key platform chemical in industry – most notably for the production of fertiliser – but the Haber–Bosch process currently used to produce it involves breaking the dinitrogen triple bond. This requires temperatures over 400°C and pressures of around 200 atmospheres, and as a result it accounts for around 1.8% of global carbon emissions. Meanwhile, the nitrates found in fertiliser runoff and sewage that escape into waterways lead to the growth of algal blooms. Nitrates that find their way into drinking water can cause conditions such as colon cancer. In many countries, the safe limit in drinking water for overall reactive nitrogen is 10–20mg/l.
Electrochemically reducing wastewater nitrates directly back to ammonia is, therefore, an appealing solution. However, environmental chemist Xiaoxiong Wang at Tsinghua University in China says that the vast majority of researchers working on this have been catalysis chemists. ‘In that case, they’re mostly [trying] to test the performance of the catalyst – whether it has high Faradaic efficiency, high conversion efficiency, things like that,’ says Wang. They usually test the catalysts in solutions that have quite unrealistically high nitrate concentrations. The problem, says Wang, is that wastewater is not a highly concentrated nitrate feedstock.
So the researchers developed a membrane structure in which iron was atomically dispersed within a dense woven carbon nanotube framework. An applied voltage drew the nitrate ions through the small pores, where they were reduced to ammonium. Previous catalysts – often precious or toxic metals – worked by reducing nitrate to nitrite. However, their new catalyst uses a novel mechanism in which four nitrogen atoms coordinate onto a single iron atom help to ‘efficiently break the nitrogen–oxygen bond to make ammonia’, explains Wang’s colleague Xuanhao Wu at Zhejiang University, China.
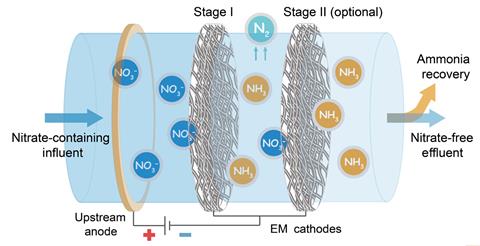
The result was a catalytic membrane that could achieve an ammonia turnover frequency of 15.1g of nitrogen per gram of metal per hour – more than four orders of magnitude higher than any previous electrochemical setup – in water containing concentrations of nitrogen as low as 100mg/l. Wang says that, in unpublished work, the researchers have developed other, simple methods to extract the ammonium ions as ammonia gas, and that they are studying ways to produce more valuable nitrogen-containing intermediates.
Chemical engineer Ke Xie at Northwestern University in Illinois is impressed by the results – although he says the researchers’ mechanistic conclusions are undermined by the fact that the membrane pores in the simulations are approximately 30 times smaller than the pores in the actual material. ‘I think it’s probably a limitation of their computational capacity, because these kinds of simulations normally can’t describe tens of nanometre scales,’ he says. He is sceptical, however, that the method will be a viable source of ammonia. ‘What you get here is 100ppm ammonium, and it would take a lot of effort to take out this in a form in which it can be used,’ he says. ‘If I wanted better performance, I’d pursue a catalyst that didn’t produce any ammonium but just produced nitrogen, which escapes.’
References
X Wang et al, Sci. Adv., 2025, DOI: 10.1126/sciadv.ads6943

















No comments yet