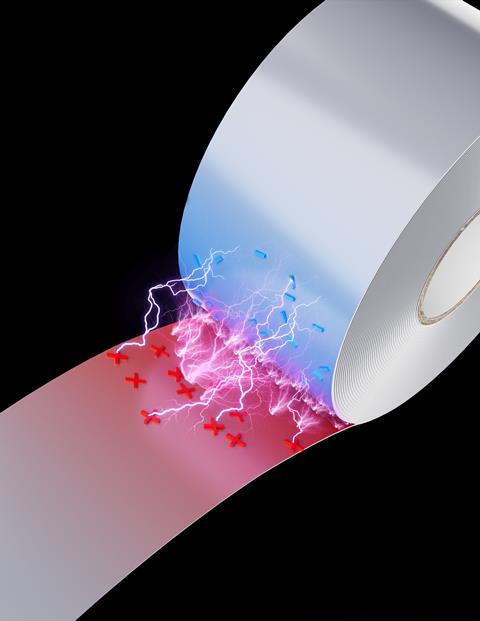
The intense electric fields generated by peeling tape can produce ‘microlightning’ that can ionise reactants and drive chemical reactions, researchers in China and the US have shown.1 The researchers believe this demonstrates the usefulness of interfacial electrical discharge in chemical reactions, potentially enabling synthesis of chemicals in greener ways than possible today. It could also provide insights into natural and potentially prebiotic processes.
In the quest to decarbonise the chemical industry, the need to design viable electrochemical replacements for processes such as the Haber–Bosch synthesis of ammonia is widely accepted. This can be achieved via arc discharge to ionise the reactants and overcome the activation energy barrier. A key challenge, however, remains generating and delivering the electricity. ‘It’s always been thought that you have to use a spark plug from a fuel-driven car or something,’ says physical chemist Richard Zare at Stanford University.
Zare and his colleagues in the groups of Xinxing Zhang at Nankai University and Tingting Zheng at East China Normal University, both in China, showed that the electrical discharge generated by peeling tape can do the job. Luminescence from the separation of adhesive tape was first observed in 1939, and in 2008 the physicist Seth Putterman and his colleagues at the University of California, Los Angeles, observed the emission of x-rays.2 This occurs because the adhesive side becomes positively charged as the tape peels away. If the tape is pulled rapidly, intense electric fields of up to a billion volts per metre can rapidly build up, leading to dielectric breakdown of the air. The resulting electric shock is partly responsible for the pain of ripping off a plaster, says Zare.
The researchers used a motor-controlled rotating roller to peel Scotch tape at a fixed speed, vibrational Stark spectroscopy to measure the intensity of the electric field at the peeling point and mass spectrometry to study the chemical species created as a result. They showed that, when peeling tape at over 0.3m/s, they could excite reactions such as the Menshutkin reaction between pyridine and methyl iodide, a cornerstone of organic synthesis with applications such as pharmaceutical production.
Putterman, who was not involved in the work, says it has obvious attractions. ‘Peeling tape has been shown to generate x-rays with energies up to 100keV. A typical chemical reaction requires energies less than 10eV,’ he notes. ‘In fact, the energies generated from peeling tape are high enough to generate nuclear fusion! There is no start-up doing this because this neutron source would be very inefficient.’ He says the overall goal is ‘wonderful’, but ‘our experience makes us very interested to hear how they plan on getting control of the discharge and fields in the small region where the fields exist’.
Zare says they don’t actually plan to use peeling tape to power reactions, but the principle that microlightning can arise at interfaces could be broadly applicable. ‘Another way is spraying water droplets, or another way is putting gas bubbles through water,’ he says. Other work just published that involves Zare, whose lead author, Yifan Meng, is also at Nankai University, details an experiment in which introducing alkyne vapours to a spray of water microdroplets led to the production of carboxylic acids under ambient conditions.3 The researchers propose a mechanism in which electrical discharge between the microdroplets leads to ozone formation, causing oxidative cleavage of the strong carbon–carbon triple bond. Zare suggests such microlightning could also be involved in natural processes such as the will-o’-the-wisp phosphorescence sometimes seen above swamps. ‘My own guess is it’s had historical purposes in maybe making the building blocks of life,’ he concludes.
References
1 X Gao et al, Proc. Natl. Acad. Sci. USA, 2025, 122, e2510504122 (DOI: 10.1073/pnas.2510504122)
2 C Camara et al, Nature, 2008, 455, 1089 (DOI: 10.1038/nature07378)
3 Y Meng, E Gnanamani and RN Zare, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c07564














No comments yet