Observation of intermolecular interactions in quinolines could help to settle the nature of this kind of bonding
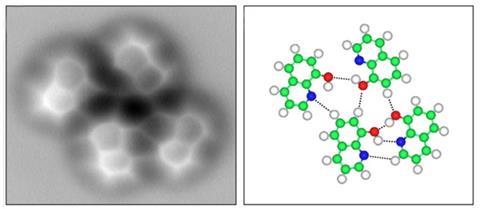
Peering at molecular structures is what chemists do. Technology that can improve the way that they see this world can have a huge impact on the field. In one such leap, researchers in China report the first visualisation of a hydrogen bond using atomic force microscopy (AFM).
In May, Felix Fischer and colleagues at the University of California Berkeley in the US used AFM to image molecules before and after a chemical transformation. The remarkable images showed the formation of covalent bonds in a cyclisation reaction.
In the latest study to visualise molecules, Xiaohui Qiu and colleagues at the National Centre for Nanoscience and Technology, China, went one step further. They used the same non-contact AFM as Fischer, but instead of looking for covalent bonds they tweaked it to look for weaker interactions.
AFM can be performed in two modes. In contact mode, the tip of an AFM made from silicon or silicon nitride drags on top of a surface. The deflection caused in the tip by the repulsive force of the surface is then processed to create an image of the surface. In the non-contact mode, the cantilever containing the tip is made to oscillate at a resonance frequency just above the surface that is to be imaged. The weak van der Waals forces of the surface decrease the resonance frequency of the cantilever. This change in frequency can then be processed to reveal the image with atomic precision.
Hydrogen bonds are fundamental to the most important molecules in nature. They are responsible for holding the two strands of the double helix of DNA together and many enzymes catalyse reactions by making use of them. These intermolecular bonds can form when a hydrogen that is bonded to a highly electronegative atom interacts with another negatively charged atom.
Despite their ubiquity, Qiu says that the ‘nature of a hydrogen bond is still debated’. It has long been considered an electrostatic interaction, but recently it has been suggested that it has chemical bonding characteristics as evidenced by x-ray diffraction experiments.
Seeing bonds
Qiu chose 8-hydroxyquinoline (8hq) to study because it is flat but its one hydrogen bond would be out of the plane of the structure, which might increase its visibility. Still, he wasn’t sure if the contrast from the hyrdogen bond would be strong enough to observe it. ‘Our AFM observations of hydrogen bonds formed between 8hq molecules were not expected, because of the very low electron density in the proximity of these weak bonds,’ he says.
For many years, an older imaging method, scanning tunnelling microscopy (STM), had better resolution than AFM. In 2011, researchers combined STM with density functional theory (DFT) to show hexamers formed by hydrogen bonding between methanol molecules adsorbed on a gold surface, albeit with poor resolution. But in 2009, Leo Gross at IBM pioneered a technique that attached a carbon monoxide molecule to an AFM’s tip, which considerably improved its resolution. This technique was used in the new study and Gross is impressed. ‘This is seminal piece of work,’ he says.
The results only confirm that AFM can be used to probe the nature of hydrogen bonds. They do not yet take the debate forward on the nature of the bond. ‘The direct observation of hydrogen bonds is consistent with the concept we learned in high school: an electropositive hydrogen atom bridging between two electronegative species X and Y in the designated form of X–H···Y,’ Qiu says. ‘The problem now is to get a deeper understanding of what causes the contrast at the positions of the hydrogen bonds,’ Gross says.
Just as chemists use NMR and mass spectrometry on an everyday basis to examine molecules, Qiu hopes that one day AFM will be a routine tool too. Gross is more sceptical as sample preparation is complex and the need for trained personnel might make it less attractive. But if these limitations can be overcome, few chemists would want to miss the opportunity to see the molecules they manipulate every day.







No comments yet