Accidental discovery shows that N-heterocyclic carbenes can act as sources of atomic carbon
Chemists in Japan have found a reaction that creates four new bonds to one carbon atom, in a single step. The reaction was an accidental discovery during experiments involving N-heterocyclic carbenes.
Many chemical reactions involve adding a carbon-containing unit to a substrate molecule. But almost none involve adding just a single carbon atom. This is mainly because of atomic carbon’s extreme instability and the lack of a simple way to generate it under regular laboratory conditions.
Now, a team led by Osaka University’s Mamoru Tobisu has developed a way to tame atomic carbon and use it as a viable reagent. ‘We found a chemical reaction that adds a single atomic carbon to an organic compound,’ says Tobisu. ‘The key to control the violent reactivity of an atomic carbon is to avoid the use of carbon atom itself and utilise a masked equivalent of it.’
Tobisu’s ‘masked atomic carbon’ is an N-heterocyclic carbene – a widely studied class of compounds containing a divalent carbon atom. While investigating one particular carbene-catalysed rearrangement reaction involving unsaturated amides, Tobisu’s team noticed that, rather than their expected product, the experiment produced a lactam containing an extra carbon.
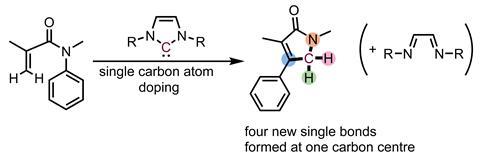
‘Later, we noticed that this reactivity is quite reasonable based on the resonance theory,’ says Tobisu. The researchers realised that the carbenes can also act as an atomic carbon coordinated to a 1,2-diimine. In their experiments, the carbene had simply donated that carbon to the amide substrate, while producing a diimine side-product.
‘The central issue of organic synthesis is how to improve the efficiency in increasing the structural complexity of molecules,’ says Tobisu. ‘Our method allows for four chemical bonds to be formed at the carbon centre in a single step – thereby shortening the chemical processes that had been required using classical methods to construct an elaborate structure.’
Since that initial discovery, Tobisu’s team has optimised the substitution pattern on the carbene to improve the reaction’s selectivity towards lactam products. They also showed that the reaction can work on several unsaturated amide starting materials featuring a variety of different functional groups.
‘The formation of new carbon–carbon bonds is the very foundation of organic chemistry. While there are many longstanding methods for carbon–carbon bond formation in the synthetic toolbox of organic chemists, Tobisu and co-workers have presented a fundamentally new method in this paper,’ comments Stacey Brenner-Moyer, an expert in synthetic organic chemistry based at Rutgers University, US.
She notes that while N-heterocyclic carbenes are commonly used as ligands for metal catalysts or alone as organocatalysts, their use as single-carbon atom transfer reagents represents a ‘remarkable’ new application. Tobisu’s group now plans to dig deeper into the underlying mechanism in the hope of finding ways to make the reaction more general.
References
M Kamitani et al, Science, DOI: 10.1126/science.ade5110














No comments yet