Previously impossible to monitor properties of transition states found hidden in vibration spectra
Every student is taught that chemical reactions go from the reactants to products via a fleeting higher energy transition state. However, the transition state only exists for the briefest moment, making it very hard to study. Transition state theory is a key part of our understanding of why and how reactions occur, but there are very few experimental studies of the transition state.
Now researchers in the US have devised a method to deduce the energy of the transition state from experimental measurements of the patterns of quantised vibrational energy levels of a molecule approaching the transition state. For the first time researchers have access to previously unmeasurable rotation constants and frequencies of the transition state.
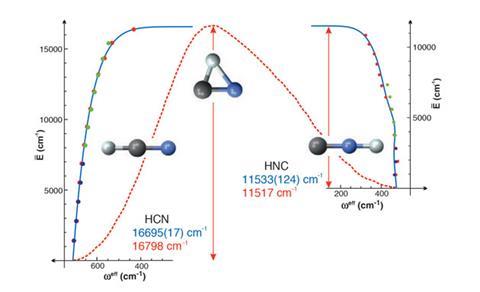
Experimental studies of transition state energies are rare, due to the very short lived nature of the transition state. ‘If you were going to get some sort of energetic snapshot of the molecule as it traverses the transition state you’d be limited by the uncertainty principle,’ explains group leader Robert Field at the Massachusetts Institute of Technology. ‘You couldn’t measure any energy at that resolution. A femtosecond is so short it blurs all the details that would reveal the spectrum or the structure of the transition state.’
Co-author Josh Baraban adds: ‘Our work was about was using spectroscopic techniques to get properties of the transition state in a completely independent way. You can get energy, information about its vibrations, information about its rotations. The kind of precise information which you can get out with our types of spectroscopic techniques has never really been available; people really didn’t even think it was possible.’
Interrogating isomers
The group studied isomerisation of an electronically excited state of acetylene. Acetylene is linear in its ground state, but when an electron is excited from the triple bond the molecule bends to be either cis or trans. Tuneable laser spectroscopy was used to study interconversion between the two conformers, and the pattern of vibrational energy levels. As the energy levels increase the patterns break down, with molecules showing vibrations at lower frequencies than expected. ‘By measuring patterns in the energy levels revealed in a spectrum we are able to both determine the energy of the top of this barrier and describe something about the structure or the shape of the molecule at the transition state,’ explains Field.
Baraban hopes that this work will eventually be scaled up to more complicated systems. ‘There are only ever going to be a few vibrations that are related to the motion that takes you from reactants to products. You can do this analysis just for those few modes – that doesn’t mean it’s going to be easy in a larger system, there are all kinds of complications, but it does mean that it’s not hopeless. I hope that people will design other kinds of experiments and other kinds of new spectroscopic techniques that might be able to scale to larger systems.’
Donald Truhlar of the University of Minnesota, US, says that the work is a ‘significant advance’. ‘Field and co-workers have made stimulating progress by showing that spectator modes need not prevent observation of interesting effects in reaction–coordinate modes.’ He warns that extending the work will not be easy. ‘A challenge in extending this method to more complex isomerisations will be finding cases where the passage between the two minima is coherent and with no vibrational decoherence due to intramolecular vibrational relaxation. In some respects that will limit it to double minima of spectroscopic interest rather than to isomerisations where kinetics can be measured and transition state theory can be applied.’
References
J H Baraban et al, Sci. Adv., 2015, DOI: 10.1126/science.aac9668











No comments yet