Molecular shapeshifting observed at ultrafast speeds
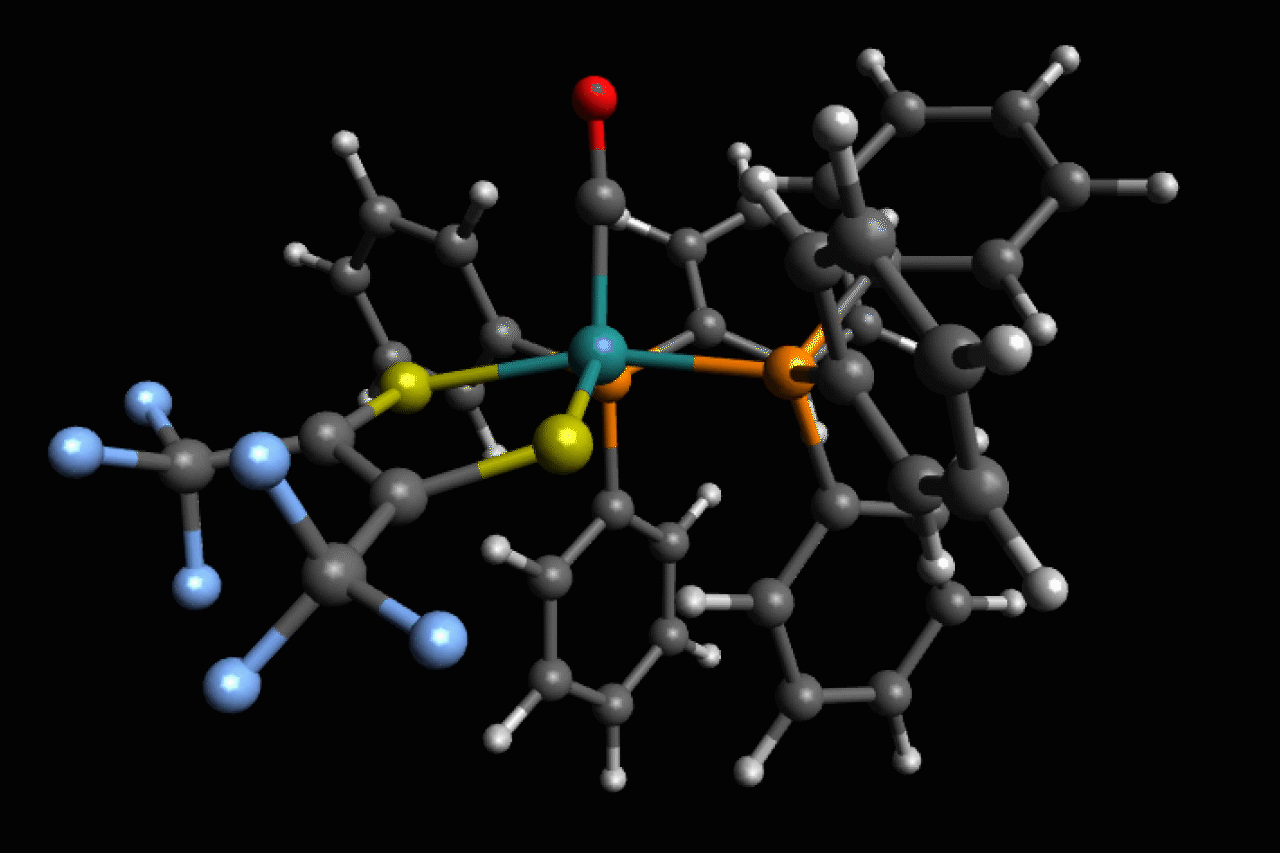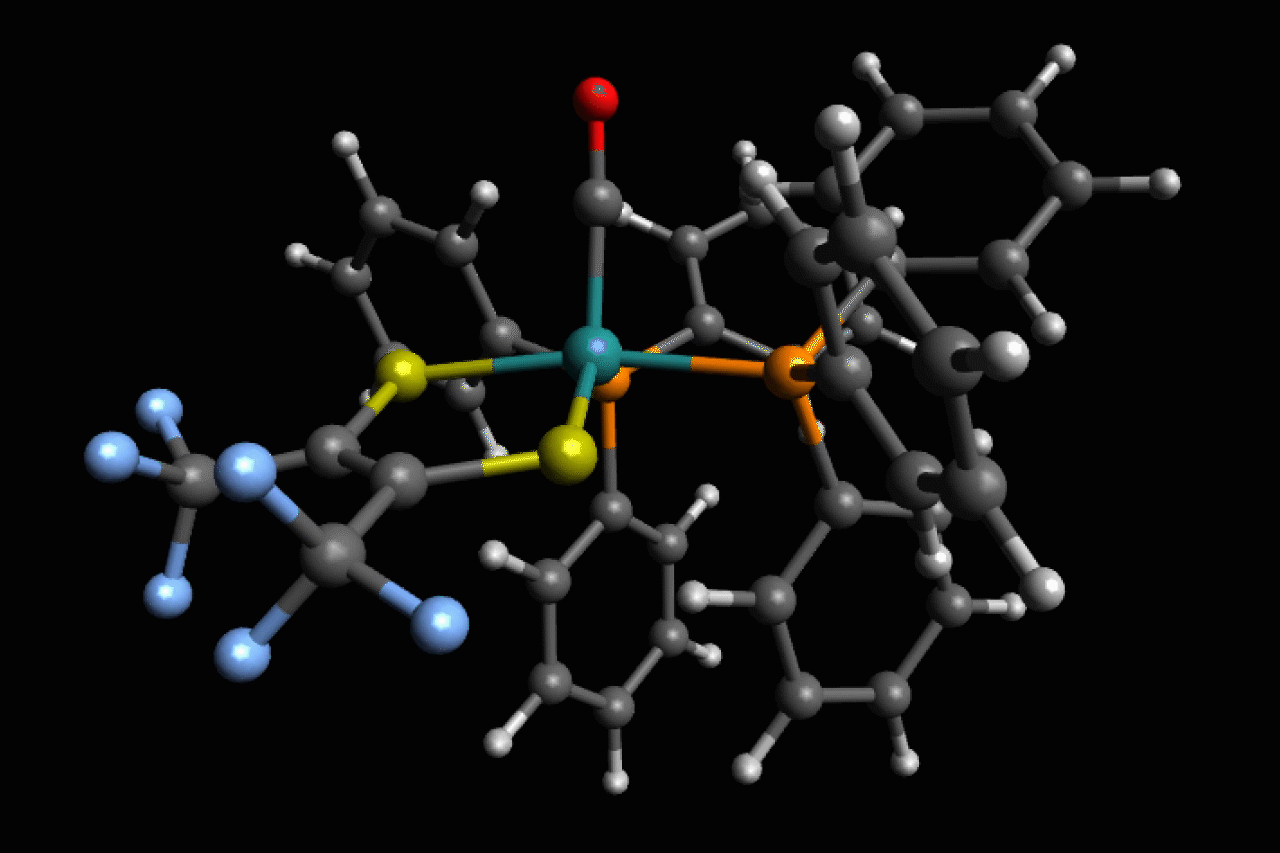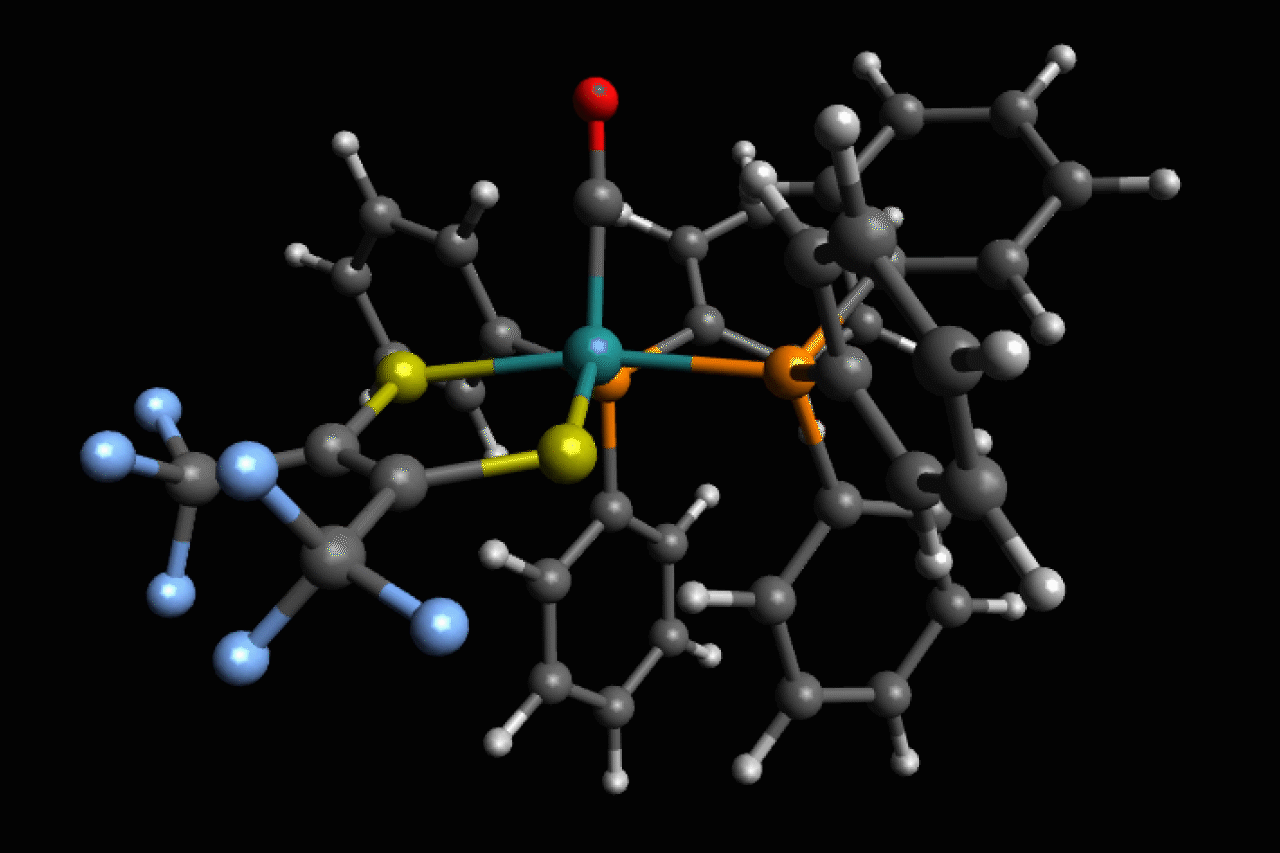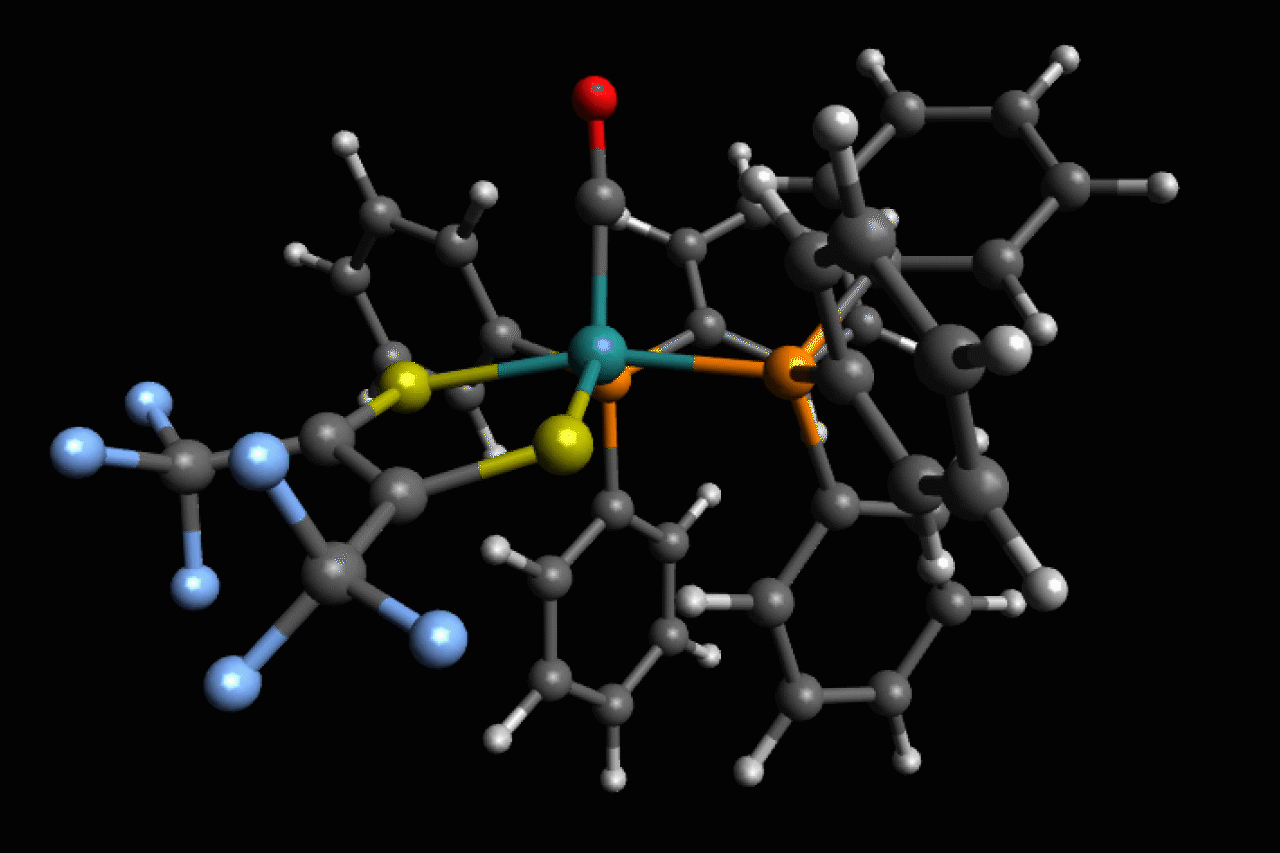
Study reveals that structural transformations occur just as fast as molecular vibrations
Scientists in the US have observed ultrafast structural isomerisations at picosecond (1 × 10-12s) speeds, adding another chapter to the story of Berry pseudorotation in the process.