A metal–organic framework (MOF) nanoribbon could underpin a new source of clean energy by harvesting the latent heat of evaporation in seawater. A Chinese team has developed a small prototype device that separates sodium and chloride ions to generate a sustained potential difference powered by this natural phase change.
Extracting energy from water evaporation could be a major boost for sustainability efforts due to the abundance of suitable locations for this technology. Evaporation-induced electricity generation relies upon the electrostatic interactions between a flowing fluid and a surface. But these methods are currently limited to pure water systems as the high concentration of ions in seawater disrupts the flow of charge.
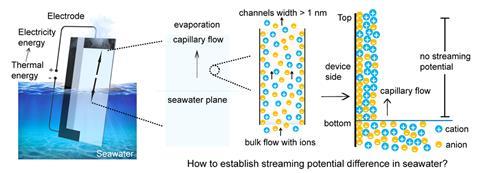
‘Within a porous medium, there exists an electric double layer between the solid surface and the fluid,’ explains Yan Lu, a battery researcher from the Helmholtz–Zentrum Berlin in Germany. Charged particles form a continuous layer over the surface and this in turn attracts a diffuse layer of counterions from the solution. ‘As the liquid flows, powered by continuous evaporation, variations in the mobility of these charged species in the diffuse layer give rise to the generation of a potential difference along the fluid’s direction,’ says Lu. ‘But in seawater, the abundant ions flow along with it, greatly diminishing the potential difference caused by the differential flow of the electric double layer.’
Now, Hongfang Liu and Bao Yu Xia from Huazhong University of Science and Technology have developed a device that retains this crucial electrokinetic effect in salt water. Their MOF, composed of calcium ions and benzene hexacarboxylic acid (BHA), forms a selectively permeable network of nanochannels that generates a potential difference between the top and bottom of the device as water evaporates through it. ‘The synthetic Ca-BHA MOF has hydrophilic and negatively charged sub-nanochannels, enabling water and sodium ions to transport but avoiding chloride ion flow, thereby generating a streaming potential,’ explains Xia. ‘This behaviour is related to the internal hydrogen bonding network structure.’
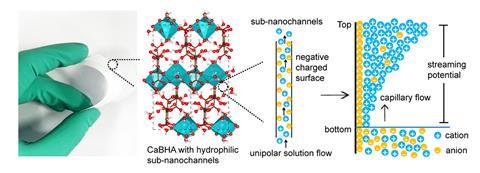
The prototype device, which measures two by six centimetres, maintained a steady voltage of 0.4V under natural evaporation conditions without any signs of degradation over a three-day period. Although still at the proof-of-concept stage, Lu is impressed by the MOF’s performance and interested to see how Xia and Liu will develop the system further.
‘This work provides a novel solution for flow-evaporation electricity generation in liquids rich in high-concentration ions,’ she says. ‘Moreover, these highly selective sub-nanometre channels hold promise for applications in other fields, such as selective separation of ions. I would like to see this work progressing towards practical and industrial applications by researching parameters in settings closer to real-world scenarios.’
At a fundamental level, the team would like to fully understand the exact ion transport mechanism within the MOF’s nanochannels. However, their current focus is on developing the prototype device into a larger practical system. ‘Commercialisation is far away,’ says Xia. ‘The next step we would like to take is to enlarge the electricity generation performance. Further evaluation is also needed for device placement, salt resistance, and performance stability.’
References
Z Wang et al, JACS, 2024, DOI: 10.1021/jacs.3c13159















No comments yet