Capturing carbon dioxide from seawater to address climate change could be cheaper and more energy efficient than existing systems that capture it from air. Chemical and mechanical engineers at the Massachusetts Institute of Technology (MIT) have used an electrochemical system to take advantage of the fact that oceans and other surface waters act as large carbon sinks that have absorbed 30% to 40% of anthropogenic CO2 emissions since the industrial revolution began.
Existing methods for removing carbon dioxide from seawater apply a voltage across a stack of membranes to acidify a stream of water by splitting it. But these membranes are expensive, and reagents are needed to drive the overall electrode reactions at either end of the stack adding to the cost.
The MIT researchers are proposing a novel approach based on electrochemical modulation of seawater’s pH to free the CO2 and then return the water to its original pH, before releasing back into the ocean. The process uses a bismuth electrode that reacts with chloride ions in seawater under an oxidising potential to produce bismuth oxychloride and protons. As the water gradually becomes more acidic the bicarbonates are converted into CO2, which can be tapped off.
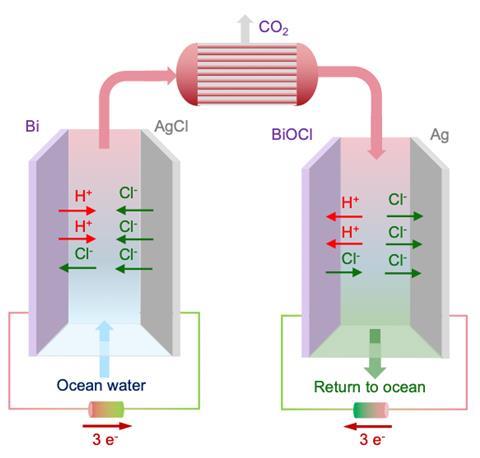
This acidified water is then shunted to the second cell, where the reactions are reversed, so that the water becomes more basic. ‘This is what we feed back to the ocean, so we’ve actually done both the CO2 removal and the alkalisation … of the ocean water,’ says Alan Hatton, a chemical engineering professor at MIT and one of the study’s co-authors. When these electrochemical cells’ electrodes are depleted they can simply be switched around in the process to regenerate them.
Releasing CO2 from ocean water can free up this seawater so that it can capture more CO2, but the alkaline water that the MIT team discharges can also be important to counteract rising acidity in the oceans. ‘The net result of what we do is to remove CO2 from the environment as a whole – anthropogenic CO2 will distribute between the ambient air and the ocean water, so … removing it from the oceans means that there is a drive for more to be absorbed and thereby be removed from the air,’ Hatton explains.
While direct air capture is a two-step process, in which the CO2 is first captured by a sorbent before further energy is needed to release it, the new MIT system skips the capture step as that has already been done by the ocean itself. The method could be more efficient than air-capture systems because the concentration of dissolved CO2 in seawater is more than 100 times greater than it is in air, Hatton notes.
He says he is ‘somewhat agnostic’ as to what to eventually do with the captured CO2 as there are several different routes for its disposal. ‘It can be used as a feedstock for fuels, chemicals and materials … or it can be sequestered in sub-surface geological formations,’ Hatton explains.
Initially at least, the idea would be to couple such systems with existing infrastructure that already processes seawater, such as desalination plants. The team hopes to eventually see this technology deployed as free-standing carbon capture plants around the world.
A preliminary technoeconomic analysis also suggests that this ocean capture system would be economically feasible, with costs ranging from $50 to $100 (£42–£83) per tonne of CO2. One pioneer of air capture technologies calculated that it cost $600 to capture 1 tonne of CO2 in 2020. The researchers are now patenting the technology.
Dan Esposito, an associate professor in Columbia University’s chemical engineering department who was not involved in the study, is enthusiastic about the work and declares it ‘all sound’. He notes, however, that the concept of extracting CO2 from seawater is not new. ‘The contribution here is that they are using a membrane-free architecture, while electrodialysis cells use bipolar membranes that are quite expensive,’ explains Esposito, who is also co-founder and adviser to start-up sHYp that is working on direct ocean carbon capture.
Esposito suggests, however, that many assumptions in the paper are ‘very rosy’, including some of the cost estimates. Nevertheless, he agrees with the MIT group that direct ocean capture is very promising, and that it may have some benefits over direct air capture from an energy efficiency standpoint.
‘The ocean has already captured the CO2, and I think that is an inherent advantage here that can translate to lower system costs,’ Esposito tells Chemistry World. ‘It is a smaller volume of water that you need to pull in and process compared to air for direct air capture systems.’
References
S Kim et al, Energy Environ. Sci., 2023, DOI: 10.1039/d2ee03804h















No comments yet