Nylon-6 can be rapidly broken down by a new catalytic process without generating harmful byproducts or requiring toxic solvents. The metallocene catalytic system, which is based on earth-abundant early transition and lanthanide metals, depolymerises the material at unprecedented rates.
Using this catalyst, the team was able to recover 99% of the nylon-6’s monomers, which could then be upcycled into higher-value products. The system is also highly selective and only acts on nylon-6. Consequently, the researchers suggest that it could be applied to large volumes of unsorted waste.
This new system is also greener than other catalysts that have previously been investigated, which rely on high temperatures, high-pressure steam or toxic solvents.
In contrast, the catalytic process developed at Northwestern University in the US doesn’t depend on expensive materials or extreme conditions. The catalytic system involves lanthanide metal ions encased in a specially designed organic ligand framework. To recycle these resilient plastics, these catalysts must be tailored to not only be highly active, but also thermally stable and robust to handle and break down these polymeric materials.
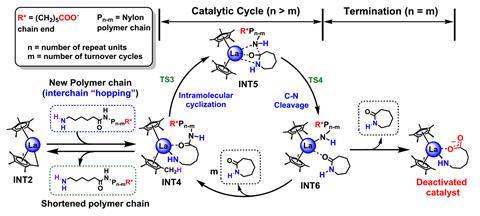
The researchers also relied on high-level quantum mechanical calculations to examine the energetics of various possible catalytic reaction pathways and help guide them in designing the catalyst.
‘You can think of our catalyst as almost a Pac-Man – it starts at one end of the polymer and chomps along it and spits out the original monomer, called caprolactam,’ explains Tobin Marks, the study’s senior author. ‘And caprolactam actually comes out as a vapour so there’s no solvent, and we collect it – we cool the vapour and it forms very pure caprolactam.’
The catalyst moves down the polymer chain, rapidly expelling the caprolactam monomer molecules one by one. Its molecules can also jump from one chain to another. ‘We show in the paper that the catalyst can be recycled multiple times without slowing down, arguing that it can be used in a continuous process in which nylon is continuously fed to the reactor and caprolactam continuously exits from the reactor as would be done in a large plant,’ Marks states.
‘We understand through these computational works that our metal catalysts bind with the amide bonds of these nylon-6 polymers and start to “unzip” these plastics with monomers releasing at the polymer chain end,’ explains Liwei Ye, the paper’s first author. ‘These computational studies are important because they guide us, as experimental chemists, how to better design our catalytic structures and to make these processes more efficient.’
The Northwestern team has demonstrated that their new catalytic process can target nylon in a mixed plastic waste stream. ‘And now we’re looking at other mixtures, so that’s the next step of our research,’ he says. ‘Interestingly, there is an increasing demand especially for high end clothing made from recycled nylon, because people want to see that environmental sustainability consciousness.’
Beyond catalysis?
Marks and colleagues report a new π-ligated class of metallocene catalysts. They hope that it can now be used on a large scale to help tackle the global plastic waste problem.
Collin Ward, a marine chemist at Woods Hole Oceanographic Institute in Massachusetts, US, who was not involved in the work but researches how plastic degrades in the environment, says the study offers ‘a creative and novel way’ to recycle nylon. ‘I’m impressed with the number of experimental variables they considered when evaluating the effectiveness of the catalysts, showing great promise of the catalyst across many important test conditions.’
The logical next step, according to Ward, is to understand how feasible it is to scale this technology. ‘If successful, I agree with the authors that the technology could increase the circularity of nylon consumer products and reduce the amount of nylon that ends up in landfills,’ he says.
Yutan Getzler, an expert in polymer chemistry and recycling at Kenyon College in Ohio, says the problem with nylon-6 recycling is not one of chemistry, but economics. There are very well-established industrial processes to recycle nylon and resell it as other products, ‘but the question of whether or not they’re operating effectively really has to do with fluctuation in the price of petroleum, which is what’s going to define the price of petroleum-derived caprolactam relative to a chemically recycled material’, he explains. ‘You just can’t get enough of it to run a process efficiently at industrial scale, and that is not a problem that’s solved by catalysis.’
Liwei Ye’s name was corrected on 6 December 2023
References
L Ye et al,Chem, 2023, DOI: 10.1016/j.chempr.2023.10.022
















No comments yet