A single polymer feedstock has been used to form distinct plastic products including cutlery and adhesives – something that is impossible with conventional polymers. The US team reversibly tempered the ‘pluripotent’ plastic at different temperatures to create particular physical characteristics on demand.
A plastic’s properties are typically determined by chemical composition and, over the last century, scientists have developed hundreds of different polymers, each suited to a particular application. However, while some materials such as polyethylene can be remoulded, their fundamental characteristics such as hardness, brittleness and elasticity remain fixed, substantially limiting the uses of each polymer.
Now, Stuart Rowan at the University of Chicago, US has developed a pluripotent polymer feedstock, enabling the team to alter the plastic’s properties through careful control of the temperature just before it’s ready to use. ‘We were inspired by stem cells and their ability to differentiate into a range of different cell types in the human body, depending on environmental cues,’ says Rowan. ‘We made a polymer network that can be tempered, similar to the process in metals where a material is heated at a precise temperature before being rapidly cooled or quenched, [to] access materials that range from being hard and high strength to soft and extensible at room temperature.’
Conventional plastics cannot be tempered: thermoplastics such as polyethylene simply melt and thermoset plastics like epoxy resins decompose. However, the team designed a dynamic cross-linked polymer network in which the covalent bonds between the chemical components can reversibly break and form depending on the treatment temperature within the material’s ‘tempering window’. Between 60°C and 120°C this reversible bond reconfiguration allows the polymer to exhibit its unexpected ‘pluripotency’. ‘With different temperatures you can actually control the number of crosslinks,’ explains Maarten Smulders, a dynamic polymers researcher from Wageningen University in the Netherlands who wasn’t part of the new work. ‘The higher the tempering temperature, the more bonds are opened up so there’s a lower degree of adduct formation which results in a softer material.’
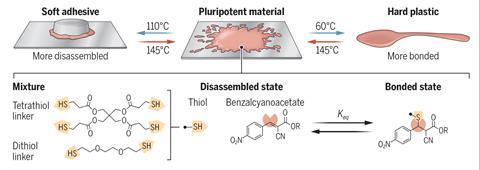
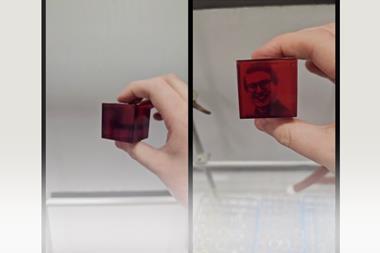
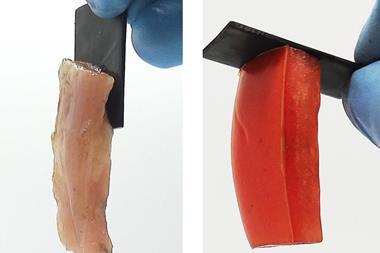
The quenching step halts this reversible bond breaking process and fixes properties in the material based on the extent of crosslinking. But the dynamic nature of these bonds means that the quenched plastic can also be re-tempered. ‘You can reverse these changes and use the same material for different applications,’ explains Filip Du Prez, a polymer chemist at Ghent University in Belgium. ‘[Rowan] has a very nice prototype – at 60°C he made a material that is good enough to work as a plastic fork or spoon. Then exactly the same material tempered at a higher temperature (110°C), he could use as an adhesive.’
Rowan’s team is conscious that further development is needed to make the new plastic commercially viable. Slow heat dissipation through the polymer leads to long tempering times and the unusual chemical components are not readily available at scale. ‘[Another] next step is to improve the range of mechanical properties that our pluripotent materials can access and to design pluripotent materials in which functional – as opposed to mechanical properties – can be tuned through tempering,’ says Rowan.
But for Smulders and Du Prez, these early results offer a hugely exciting opportunity for polymer chemists, while also raising a number of intriguing questions. ‘It’s really interesting that they could maintain these properties at the quenched material’s operating temperature,’ comments Smulders. ‘I would also love to learn more about the exact factors which produce this tempering window and drive the change in mechanical properties.’
‘This has opened my eyes and might lead to a whole new generation of materials making use of dynamic chemistry,’ says Du Prez. ‘I’m thrilled to see what the future will bring and a big question for me is can we start to predict which other materials can show this property?’
References
NR Boynton et al, Science, 2024, 383, 545 (DOI: 10.1126/science.adi5009)















No comments yet