A new spectroscopy technique allows researchers to map out hydrogen-bonded networks and study how changes in conditions affect them. The technique, which is derived from the long-known hyper-Raman scattering, provides new information about bulk water and could provide direct experimental insights into a wide variety of problems that, until now, could only be addressed computationally.
Hydrogen bonds are responsible for the anomalous properties of water. The precise nature of the hydrogen bond remains somewhat mysterious, mostly because direct experimental interrogation of hydrogen bonds is challenging. Direct information about hydrogen bonds comes from their stretch mode. ‘This is the displacement of water molecules along the hydrogen bond, and it’s therefore exactly what you need to probe hydrogen bonds,’ says Sylvie Roke at EPFL in Switzerland. However, measuring the excitation is extremely difficult as the spectral region is hard to access and crowded with numerous other low-energy excitations.
Roke and her colleagues at EPFL turned to hyper-Raman scattering, a non-linear spectroscopic technique first developed in 1965 but not widely used, to measure the signal directly. ‘I’ve always asked myself, “Why would somebody do this spectroscopy?”’ says Roke, ‘Because basically you measure either the IR or the Raman vibrational modes, which is nice, but the interpretation is a lot more difficult.’ However, using symmetry considerations the researchers worked out that, if they recorded four hyper-Raman spectra of each sample, changing the position of the detector and the polarisation of the light, they could measure separate spectra of interacting and non-interacting molecules. ‘It’s a matter of adding and subtracting the spectra, and it provides information previously only accessible through a computer,’ says Roke.
Direct measurements
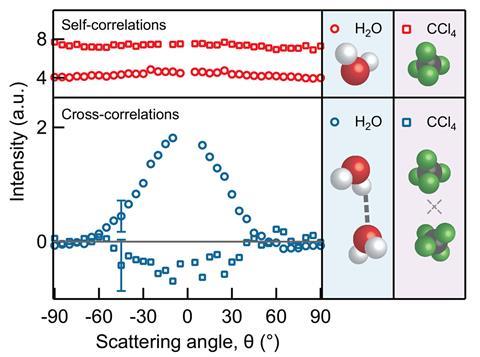
The researchers validated their technique, which they named correlated vibrational spectroscopy (CVS), by recording spectra of both water and tetrachloromethane. Water, with significant hydrogen bonding, has a CVS spectrum for interacting molecules, showing a hydrogen bonding stretching mode. Tetrachloromethane – a fluid of almost completely non-interacting molecules – produced a flat CVS spectrum. They then used deuterated water to study the effect of the quantum delocalisation of the hydrogen nucleus – which is smaller in deuterium – and how changes in pH affect the transfer of electronic charge into the hydrogen bonded network. They found both effects are mixed. ‘We don’t see much of a nuclear quantum effect when we change OH- to OD-,’ Roke says; ‘If we change H3O+ to D3O+ we see a big shift, and the effect of the hydrogens is to reduce the amount of charge transfer.’ Collaborators in France, Italy and the UK performed computations to interpret the differences.
The researchers believe the technique has huge potential. Roke points specifically to the possibility of resolving long-standing arguments about water’s role in protein denaturation. She also notes the potential to study interactions in other liquids or amorphous materials. ‘There’s almost an infinite amount of things you can do for which you would previously have had to go to a computer, ask “What happens if I do this and this?” then make a measurement and hope the two could somehow be connected,’ says Roke.
Chemical physicist Anders Nilsson at Stockholm University in Sweden believes the researchers have already produced important findings using the new technique: ‘In particular, the results on the asymmetry in charge distribution to the hydrogen bonds of solvated OH- and protons – that the former is much more delocalised, involving three hydration shells, are surprising,’ he says. ‘It has been known for several decades about the importance of nuclear quantum effects – that they lead to a weakening of the hydrogen bonds in the network – but now we have numbers from the new data from a comparison between H2O and D2O. This information will have implications for our understanding of water.’
References
M Flór et al, Science, 2024, DOI: 10.1126/science.ads4369
















1 Reader's comment