Scientists from the US and South Korea have developed a probe that can detect the highly toxic chemical hydrazine in a variety of different environments, including living cells.
Hydrazine plays an important role in a number of industrial processes. It’s used in pesticides, in nuclear and conventional electric power plants to reduce corrosion, and as a gas-forming agent in air bags. It’s even found in rocket fuel.
Although contact with small amounts of hydrazine is unlikely to cause harm, long term exposure can damage the liver, kidneys and central nervous system. Hydrazine has also been classified by the US Environmental Protection Agency (EPA) as a probable carcinogen.
Accidental leakage of hydrazine into the environment is rare and as hydrazine breaks down rapidly in oxygen, finding high levels of hydrazine in the environment is unlikely. However, hydrazine exposure in the workplace can be a real danger for individuals who come into contact with it. This makes the development of hydrazine sensors an important area of research.
The sensor developed by Jonathan Sessler from the University of Texas at Austin, US, and colleagues, uses a naphthalimide derivative which binds specifically to hydrazine and undergoes a hydrazine-induced cyclisation to give a fluorescent response as well a visible colour change. It was demonstrated that this fluorescent response could be used to detect hydrazine in water and in air, but most remarkably, the sensor was sufficiently bio-compatible for use in living cells. Sessler explains that this will enable them to ‘monitor toxic events associated with hydrazine exposure in real time’.
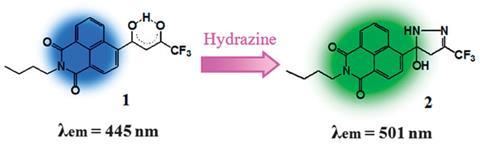
A key advantage of the probe is that it selectively detects hydrazine over other competitive compounds such as ammonia or other amines. The sensor is very sensitive too, with a detection limit of 3.2 parts per billion (ppb), well below the exposure limit of 10 ppb set by the EPA.
Tony James, an expert in sensor design at the University of Bath in the UK, describes the new sensor as a game changer. ‘The sensor is simple, sensitive, and highly selective and could be used in a variety of environments – from dip-stick tests right through to cellular imaging.’
Sessler’s team is now developing nanoparticle supports to improve the usability of the sensor.







No comments yet