A chemical redesign turns a promising bio-derived polyester into a fully recyclable polymer that exhibits record thermal stability. These structural modifications address key issues that have previously limited the use of polyhydroxyalkanoates (PHAs) as commercial plastic alternatives.
PHAs, which are produced naturally by several bacteria, are a class of polyester that are promising candidates for new sustainable plastics. ‘The presence of ester bonds offers more possibilities for chemical recycling than polymers built around a hydrocarbon backbone,’ explains Antoine Buchard, a sustainable polymers researcher from the University of Bath, UK.
Due to their tuneable properties and biodegradability, PHAs could help to tackle the global plastic waste problem. However, several fundamental challenges have limited their commercial use. The two hydrogen atoms adjacent to the ester bond are particularly labile and readily eliminate at elevated temperatures. This thermal instability is particularly problematic during melt processing, when plastics are shaped and moulded. And although PHAs are biodegradable, there are no effective methods to completely recover the monomer units, meaning valuable chemical feedstocks are lost to the environment rather than reincorporated into new products.
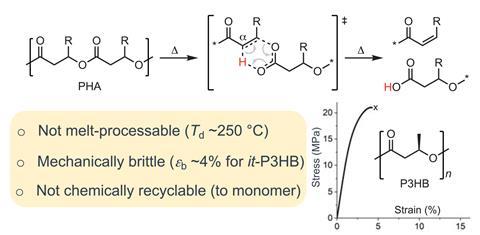
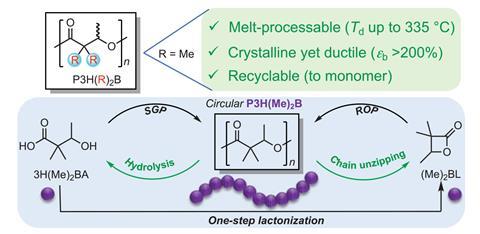
Now, a team led by Eugene Chen at Colorado State University, US, has developed a synthetically modified PHA that demonstrates both high thermal stability and complete chemical circularity. ‘We took a fundamental approach to substitute the two labile hydrogen atoms at the α,α-position to prevent decomposition by cis-elimination at high temperatures,’ he explains. ‘This alkyl substitution drastically enhances the thermal stability so the resulting PHAs can now be melt processed. They are also mechanically ductile and tough, even outperforming high density polyethylene and isotactic polypropylene.’
This same structural alteration also enables complete depolymerisation to either of the two possible monomers under appropriate catalytic conditions. ‘The addition of alkyl groups promotes a gem-disubstituted Thorpe−Ingold effect which, under certain conditions, encourages ring closure and depolymerisation of the polymer back to the original cyclic monomer,’ says Buchard, who was not involved in the project . ‘A second pathway, taking advantage of the ester linkages, is through hydrolytic depolymerisation into hydroxy acids.’
Michael Shaver, an expert on sustainable materials from the University of Manchester, UK is impressed by the work. ‘It’s an exciting first step that opens many doors. While it’s great to see the team exploring larger scales, scaling up the polymers to commercially relevant amounts is an obvious next step,’ he says. ‘The monomer synthesis procedures need improvement – both from the sourcing of the original hydroxy acids, and the route requiring a benzene sulphonyl chloride. But this work has transformative potential and it could be a real game changer if the synthesis can be improved.’
The monomer syntheses are exactly what the team plans to focus on next, in the hope of developing more efficient biological and chemical routes to the feedstocks. ‘We’re aiming to make the entire PHA production process more cost effective and economically competitive,’ Chen says. ‘We’re also engaging with industrial partners for evaluation and scaling up efforts.’
References
L Zhou et al, Science, 2023, DOI: 10.1126/science.adg4520











No comments yet