Natural biosynthetic pathways captured to produce polyunsaturated fatty acids

German scientists searching for a sustainable source of medically important polyunsaturated fatty acids (PUFAs) have shown they can be manufactured by soil-dwelling bacteria.
Research is ongoing to pinpoint who would benefit most from taking a fish oil supplement but there’s no denying that PUFAs – the good fats in fish and fish oil – have clear health benefits. However, overfishing, climate change and ocean acidification have left global fish populations, and supplies of high quality fish oil, in decline.
Rolf Müller and colleagues at Saarland University have identified that certain species of myxobacteria, also known as slime bacteria after the slime they produce to aid their movement, have the genes to synthesise certain omega-3 long-chain PUFAs de novo by employing enzymes known as PUFA synthases.
The multienzyme systems are encoded by PUFA biosynthetic gene clusters. Müller’s team have so far discovered two distinct PUFA pathways: Sorangium cellulosum can make linoleic acid and the recently discovered genus Aetherobacter turned out to be prolific producers of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ‘The presence of different types of PUFA biosynthetic pathways with such product diversity within the same bacterial family is an outstanding aspect in the field of research on PUFA biosynthesis,’ says Müller.
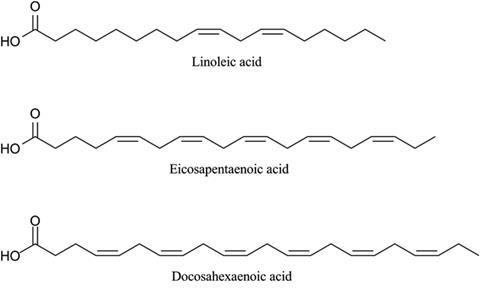
The myxobacterial pathways differ from marine systems in terms of gene organisation, catalytic domain arrangement and sequence identity of the encoded PUFA synthases. Notably, a unique domain, which most likely acts as a 1-acylglycerol-3-phosphate O-acyltransferase was identified in these myxobacterial PUFA synthases for the direct transfer of the synthesised fatty acid chains from the PUFA synthase into lipids.
The native producing strains grow slowly and are difficult to handle but the team report that the genes can be transferred to and expressed by Myxococcus xanthus, a fast growing model strain of myxobacteria.
Natural product experts praise this example of capturing natural biosynthetic pathways to produce high value-added materials. Joern Piel of ETH Zurich in Switzerland says ‘these results increase the significance of myxobacteria as remarkably prolific sources of biotechnologically relevant chemicals. The extension of available PUFA synthase types and sources by this work provide interesting opportunities for metabolic engineering.’
Craig Townsend of Johns Hopkins University, in Baltimore, Maryland, US, says the discovery of gene clusters from Aetherobacter that can produce desirable PUFAs significantly better than other microbes is important from two points of view: ‘First is the question of how catalytic domain organisation and internal kinetics in large, type I enzymes affect their product profiles, and, second how can these differences be exploited, or engineered, to lead to an economically feasible process. For EPA and DHA an important step in that direction has been made.’







No comments yet